To kick things off: I saw this picture the other day of a foot after soaking in a wet boot for 3 days. Picture turned B/W to spare you most of the gore, but by the amount of white, wrinkly mess you can tell it's not pretty:
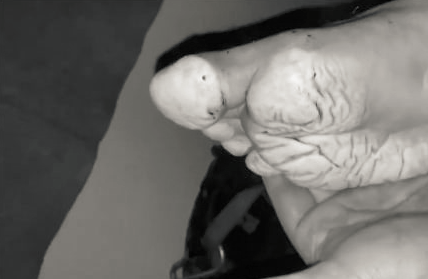
Assuming we'll need a portable implementation, how does one dry shoes from inside without taking them off?
On the surface, there appear to be a couple of methods to choose from to remove moisture:
- desiccant pads
- pumping air with lower relative humidity into an insole or inner shoe
- membrane dehumidification
- Joule heating
- electrolysis
- super-absorbers
None of the above is immediately suitable, and some may even be entirely insufficient for the task.
Requirements
Water in shoes can originate from
- inadequate clothing during rain
- sweat due to baseline environmental conditions (temperature, relative humidity)
- sweat due to physical exertion
- water ingress
and is stored in the fabric, inner shoe or insole, and perhaps to a lesser degree in calluses - all of which need to be dried as well.
With rain and water ingress subject to other mitigation measures or treated as intermittent with the ability to recover from, one thus needs to be able to extract a steady flow of water as excessive humidity or micro-capillary condensation. How much?
[1] states,
"Two key observations emerged. First, sweat secretion from the experimental foot averaged 30 ml · h−1, peaking in the last 5 min at 50 ml · h−1. Second, approximately 70% of the measured sweat flow emanated from the upper skin surfaces, with only 30% coming from the plantar surface."
As fancy as it may be, membrane dehumidifiers can be immediately excluded, as they only manage to remove 0.1 ml/dm²/h. They also do not appear to be amenable to pleating and may disintegrate after the first few 100 km of walking.
Desiccant packs are not great either - silica gel can store up to 37% water (wt/wt), while zeolite desiccant materials are around 5-35% wt/wt.
Super-absorbent materials can bind up to 500 times their weight in de-ionized water, and up to 50 times their weight when it comes to saline solutions. Sweat is salty, which could however be handled by using a short-lived membrane and diffusion. The search for "hygroscopic superabsorbent materials" yields some results [2] that are closer to 1:1 ratio and not intended to work with moisture alone, instead calling for de-ionised water immersion.
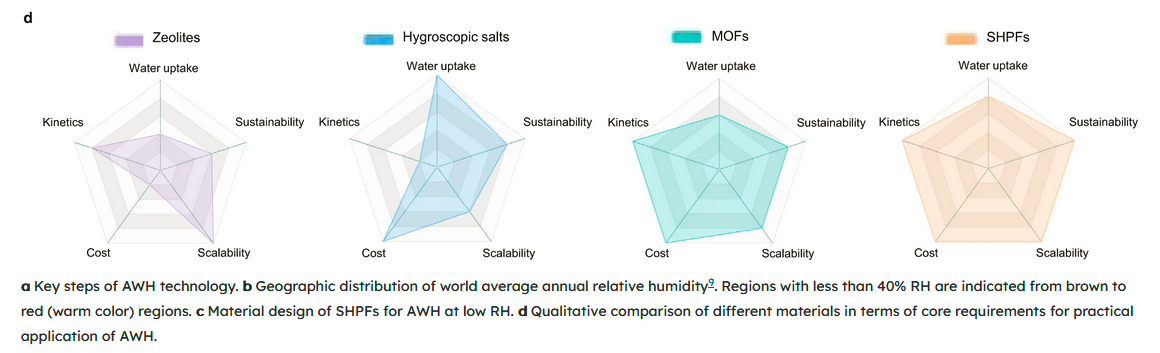
In [3], "super hygroscopic polymer films (SHPFs) composed of renewable biomasses and hygroscopic salt, exhibiting high water uptake of 0.64–0.96 g g−1 at 15–30% RH" are explored and compared. It is further stated that, "The key steps of atmospheric water harvesting (AWH) involve moisture capture and water release, followed by a simple filtration or purification process (Fig. 1a). The earlier approaches, such as fog capturing5,6 and dew condensation7,8, require the presence of high relative humidity (RH) (>90% RH), which is not a viable solution considering more than one-third of the global terrestrial area has an average annual humidity less than 40% (Fig. 1b)9."
Spiderweb diagrams for classes of desiccants are given in Fig.1, with the presented SHPFs on the right having the most favorable properties:
 The desorption however is conducted at 60°C and low rel. humidity. Fig. 3 presents the key metrics - total water uptake and vapor sorption rate:
The desorption however is conducted at 60°C and low rel. humidity. Fig. 3 presents the key metrics - total water uptake and vapor sorption rate: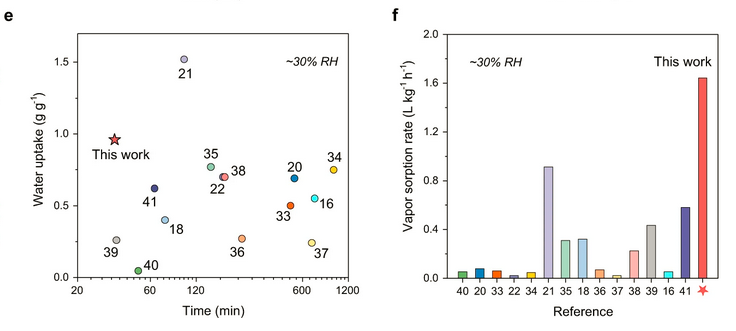
This material looks indeed rather nice if one were to cycle air through it, and probably make it hot-swappable in small canisters for regeneration at a later time.
What are the prospects of simply pumping air through an inner shoe and thereby removing moisture? The specific heat of moist outside air [4] is close to 1 kJ/kg/K for ambient temperatures, with a density of around 1.2 g/L. When drawn in from a 0°C environment and heated to 30°C, this requires 36 Ws to heat. at 10-100 ml / s, this is a delightfully low power level suitable for Li-Ion battery operation.
 Moist air at 20-25°C or higher during summer can however not remove any moisture at all, and additional heating may be required, which will be uncomfortable or induce sweating.
Moist air at 20-25°C or higher during summer can however not remove any moisture at all, and additional heating may be required, which will be uncomfortable or induce sweating.
Finally, in [5] a hygroscopic electrolyte electrolysis cell is presented which should serve to produce hydrogen with moist air as feedstock. 95% coulombic efficienc is cited, along with ~2.5 V operating voltage and considerably high current density. The idea of producing a small amount of hydrogen and oxygen which will diffuse out of the fabric seems intriguing. The layers of fabric could act as a flame arrestor, if we're lucky, but that needs to be verified experimentally. After all, the oxygen concentration is increased and the fabric materials are inflammable (cue Apollo 1). On the other hand, topical pure oxygen treatment has desirable properties, counteracting infection and improving wound healing [6].
When running the numbers however, ~96485 C/mol, 18 g/mol and 2.5 V cell voltage means it takes 13.4 kJ/g to make the water go away. That's ~3.7 Wh/g, meaning one 18650 Li-Ion cell (10 Wh) can suck out about 3 g in good operating temperatures, and a pack with 4 cells will last for 20 minutes at light / moderate exercise.
This should also give an idea of what Joule heating to dry shoes means. At 10s of watts, the level of heating will be unacceptable and probably dangerous, leading to burns when not properly monitored.
Wrap-Up
The literature research above and some back-of-the-envelope calculations suggest a dry air system may yield a rugged and low-tech system that is non-toxic, comes with acceptable power requirements and does not require opening the shoe to swap the absorbent medium.
Under resting conditions, One can probably switch to electrolysis, as this method produces no noise or vibrations and may even offer medical benefits, aiding skin regeneration and killing of pathogens.
It's time for experiments now. As far as simple aeration is concerned, I spent a few hours with boots and an aquarium pump. As a first data point, I am not entirely convinced now that simply pumping air into the inner shoe is sufficient to properly dry socks and feet. Higher flow and a desiccant cartridge which can be regenerated seem rather attractive.
Air-to-air heat exchangers might also play a part in the solution, recovering a portion of the heat when operating in colder climates.
 (picture for illustration purposes only. The membrane pump is rated at 3.7 W.
(picture for illustration purposes only. The membrane pump is rated at 3.7 W.References
- Taylor NAS, Caldwell JN, Mekjavic IB. The sweating foot: local differences in sweat secretion during exercise-induced hyperthermia. Aviat Space Environ Med 2006; 77:1020–1027.
- Li, Yun-Long; Ke, Ai-Ru; Lin, Song-Bai; Ouyang, Na (2011). Synthesis and Properties of Superabsorbent Hygroscopic Materials Based on Polyacrylamide and Poly(2-acrylamido-2-methyl Propyl Sulfonic Acid) Co-polymer. International Journal of Polymeric Materials, 60(14), 1164–1177. doi:10.1080/00914037.2011.557806
- Guo, Y., Guan, W., Lei, C. et al. Scalable super hygroscopic polymer films for sustainable moisture harvesting in arid environments.
Nat Commun 13, 2761 (2022). https://doi.org/10.1038/s41467-022-30505-2 - https://www.researchgate.net/publication/271937026_Study_of_thermophysical_properties_of_a_solar_desalination_system_using_solar_energy
- https://www.nature.com/articles/s41467-022-32652-y
- https://www.ncbi.nlm.nih.gov/books/NBK574579/
 helge
helge
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.