I'm going to admit defeat. My last hope to get the car started was charging a NiMH battery of 4 AA cells (previously shorted) with a LiSOCl2 cell of 1.5Ah capacity.

The NiMH cells read 1.33V after charging, which doesn't mean much. Figuring that not much charge had found its way into the cells, I decided to start with the capacitor at 11V. If I could boost this to around 14, start the engine, then show that the capacitor voltage was still above 11V, I'd consider it successful.
Here's the log of the capacitor voltage:
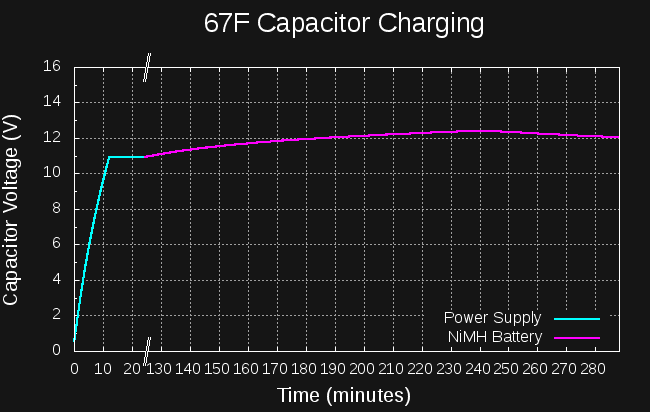
The initial almost-linear part was charging with my lab power supply at 11V, current-limited to 1A. The voltage climbed to 11V and stayed there (it's not quite linear probably due to resistance in the wires). After about an hour, I disconnected the lab supply, and put the caps on the boost converter driven by the NiMH cells (having been charged from the "coin cell"). I saw that it was working OK, and left to do some other things.
When I returned, I found that the capacitor voltage had peaked at 12.4V, then started dropping again. The NiMH cells were depleted. This means that 1097J had been deposited in the capacitor by the NiMH cells. Previous measurements had shown that 1500J are required to crank the engine.
So, it's not going to happen from one cell.
I have some more NiCd cells that were charged from LiSOCl2 cells and CR2477s, so I'll see if I can get the cap charged using the energy from multiple cells...
 Ted Yapo
Ted Yapo
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.
I wonder if a Smart car or hybrid, or any of those cars that shut off at stop lights have drastically different crank energy?
Are you sure? yes | no
The smaller the engine, the less starting energy, I'm pretty sure. The only engine I had at my ready disposal was a 4.7l V-8, which I'm sure takes more energy to crank than a 4-cylinder (or 3, even). If my snowblower starter wasn't AC-line powered, I'd even give that a shot.
Are you sure? yes | no
NO...Don't admit defeat! I think the chemical composition of the NiCd cells are your problem...too many charges and chemical breakdown...remember battery memory is a problem with cadmium.
could be a problem?
Are you sure? yes | no
Maybe. I've read that charging with very low current doesn't work well for some reason. "Trickle" charging for NiCd's is usually C/10, but from a coin cell, you can't draw anywhere near enough power to reach even that level.
Maybe you need a lot of really small NiCd cells, small enough that tiny currents can charge them efficiently, then swap them out as they get charged.
I'm only publicly admitting defeat for the coin cell challenge. This will be one of those problems that keeps me up nights...I'm sure there's a way to make it happen, somehow.
Six weeks isn't quite long enough to do the impossible :-)
Are you sure? yes | no