During my search for cheap and easily accessible rocket fuels I stumbled over Hexamethylenetetramine, also called Hexamine. Hexamine is a white crystalline compound, which is highly soluble in water and polar organic solvents. It sublimes in a vacuum at 280 °C. Together with 1,3,5-trioxane, hexamine is a component of hexamine fuel tablets used by campers, hobbyists, the military and relief organizations for heating camping food or military rations. It burns smokeless, has a high energy density (30.0 MJ/kg), does not liquefy while burning and leaves no ashes. Hexamine has been used in colored fireworks compositions as a low-reactivity, accessory fuel.
But unfortunately I discovered that hexamine is not oxidizable by KNO₃, NaNO₃, LiNO₃, RbNO₃, Ba(NO₃)₂ and probably all other alkali or alkaline earth metal nitrates:
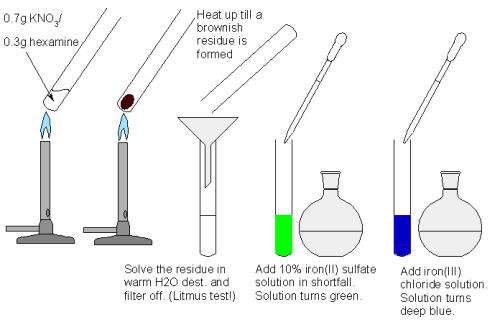
Hexamine reacts though violently with AgNO₃ but silver nitrate is not an option. A hexamine/KNO₃ mixture form cyanide's, highly basic compounds among others when enough energy in form of heat is applied, but does not burn. There are many color reactions used in cyanide determination. I have used the classical Prussian blue method:
 M. Bindhammer
M. Bindhammer
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.