-
Inexpensive Proton Exchange Membranes
08/10/2018 at 19:38 • 0 commentsHere is a research update for the project
https://www.sciencedirect.com/science/article/pii/S2405653716300835 - super info
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4385132 cabon as electrode material
increasing surface area = increase mah output
terra cotta as a pem, it looks promising BUT there are many details that are not avail to do this as a DIY home project and in the efforts of OPEN and DIY I will stick with more avail materials
https://link.springer.com/article/10.1007%2Fs00449-013-0967-6
Study on Agar as a electolytic / PEM
https://aip.scitation.org/doi/10.1063/1.5029150
A BI POLAR membrane, two membranes in dual chamber with center transitional chamber
https://www.tandfonline.com/doi/full/10.1080/21553769.2016.1230787
-
Hacking Low Cost Ceramic for Membranes in Fuel cells
08/04/2018 at 22:13 • 0 commentsSo I have been doing a bit of reading on various materials. There have been studies conducted on earthenware membranes. This was a clever suggestion from Mechanicus. Terra Cotta is one of these. Though I could spend lots of money on custom made film membranes, in the spirit of hackaday I need to HACK something that is both common , repeatable and effective. I believe earth based/ clay membranes would be much better as the cost is less than 10 percent that of a designer membrane from somewhere like fuelcellstore.com for example.
![]()
36 dollars plus tax for 11 sq ft of PEM / Cation Membrane material is a fantastically priced over the designer films
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4744959/
http://www.clays.org/journal/archive/volume%2012/12-1-397.pdf
![]()
![]()
![]()
![]()
![]()
![]()
![]()
https://www.hindawi.com/journals/tswj/2015/864568/ good article related to my setup
-
Tiny update, Researching the next cell design
08/01/2018 at 13:17 • 0 commentsNo building or design as been done at this time while another collaborator and myself research the logical next steps. We are coming up with options for Proton Exchange membranes. Namely what we would like to so is have a membrane that satisfies more than on need or a system that takes advantage of CO2 creating bacteria to feed the requirements of the micro algae on the other side of a cell. Some research must be done to determine is the electrode itself must be in plain view of light.
-
3 weeks Algae Growth
07/27/2018 at 01:19 • 0 comments![]()
A picture doesn’t do it justice because my phone keeps raising the contrast of the picture thinking it is too dark when the algae is centered in the frame. Without this significant brightness increase you can’t see the tubes inside the liquid. Shortly I will get a second Algae Container and Split the current sample I have so I can make a bigger setup in the future for version 2 of the semi permeable membrane upgrade to the design.
-
researching phase two, Permeable membrane use
07/24/2018 at 22:48 • 0 commentsIntroduction
Microbial fuel cell is the promising technology for clean power
generation and has gained lot of attention in recent years. Нe algae
obtained aіer MFC utilization can be further used for the production
of biodiesel, green diesel or bioethanol [1,2]. Complete utilization of
biomass is important for the transition to biofuels and bioeconomy [3].
Microbial fuel cell (MFC) consists of an anodic and a cathodic
chamber, separated by a proton exchange membrane (PEM). At anode,
organic compounds are oxidized by the microorganisms and electrons
move towards cathode through an external circuit and combine with
an electron acceptor (mainly oxygen) to generate electricity. At
cathode, oxygen is supplied continuously by aeration which is an
energy consuming process. In MFCs, mediators can accelerate the
process of electron transfer [4]. Recently, the concepts of MFCs have
been extended into dLوٴerent technologies such as Bio-Electrochemical
Systems (BESs) which produce numerous useful products such as
formate, [5] methane [6] and acetate [7]. Нe microbial desalination
cell is the most attractive option as this can be successfully
implemented for power generation, treatment of wastewater with the
simultaneous desalination of water [8]. MFCs can be used for the
production of hydrogen gas [9], powering environmental sensors and
digital wrist watch, charging a mobile phone, smartphone and LEDs
[10-13]. MFCs are also coupled with solar cells for power production
[14] which provides opportunities for utilizing solar energy in this
field. Future applications of MFCs may include their use in human
systems as well.
Utilization of microalgae in MFCs has gained interest as
phototrophic microalgae act as biocatholytes as the oxygen produced
by them serves as final electron acceptor, minimizing the energy
needed for the aeration at the cathode. Earlier work from author's
laboratory was reported on the H2 production from algae and its
utilization in carbon fuel cells for power generation [15]. Later work
involved the studies of the potential of power generation from algal
MFCs [2]. In the present study, potential of two strains of a green
colonial hydrocarbon (petroleum oil) rich microalga, Boryococcus
braunii, has been analyzed for its application in MFCs while
generating biomass for oil production. In these MFCs, green algal
strains have been used at the cathode as the source of photosynthetic
oxygen as electron acceptor, whereas sugar industry wastewater with
activated sludge, S. cerevisiae culture alone and S. cerevisiae with
supplementation of methylene blue (350 mg/L) was used at the anode.
НLs technology has great potential to couple the algal and yeast
biomass cultivation for the oil and bioethanol production, respectively,
along with wastewater treatment and power generation. Algal biomass
obtained from MFCs may be used for the production of biodiesel and
for bioethanol production through hydroliquefaction.Materials and Methods
Experimental organisms
Two strains of B. braunii, Udaisagar strain from Udaisagar lake,
Udaipur Rajasthan, India and Loktak strain from Loktak lake,
Manipur, India, were isolated by serial dilution method and plating on
the solLdLfied Chu-13 medium [16] which were grown and maintained
under controlled conditions in an incubator. Saccharomyces cerevisae
(Bakers's yeast), obtained from Indian Institute of Technology, Delhi,
New Delhi, India was revived by adding 1.3 gm yeast powder in 1 L
Sayed et al. medium [17] for 16 h at 27°C temperature in dark.
MFC design and operation
Нe MFC contained two chambers, an anode and a cathode, each of
250 ml capacity with 6.5 cm diameter and 12 cm length and with
electrode area of approximately 30 cm2
. Both the electrodes were made
up of carbon and separated by a proton exchange membrane (1afion).
A digital multimeter (Haqyue) was connected to the system for
continuous measurement of voltage (V) and current (I) at the external
load of 100 Ω. Peak values of current density (µA/cm2
) and power
density (µW/cm2
) were calculated from the values of V, I and A (area
of electrode). In MFCs under study, five sets of catholytes examined
separately with the two strains of B. braunii were
• Algal strains in Chu-13 medium;
• Algal strains in Chu-13 medium supplemented with 0.5 M NaCl;
• Algal strains in simulated sugar industry wastewater;
• Algal strains in simulated soap industry wastewater;
• Algal strains in simulated treated sewage water.
Whereas the three anolytes used were, S. cerevisae culture in Sayed
et al. medium [17]. S. cerevisae with 350 mg/L methylene blue (MB) as
mediator and sugar industry wastewater (198 ml) (obtained from
Saraswati Sugar Mills, Yamunanagar-135001, Haryana, India) with
activated sludge (22 ml) (obtained from the sewage treatment plant
Vasant Kunj, New Delhi, Delhi-110070, India). Нe experiment was
conducted in an incubator under controlled conditions of 27 ± 1°C
temperature, 1.2 ± 0.2 klux light intensity and 16L:8D light:dark cycle
to support microalgal growth and photosynthesis for 21 days which is
the best harvesting time for B. braunii strains.
Figure 1: Schematic diagram of the experimental set up of microbial
fuel cell with algal growth chamber as the cathode and yeast or
sugar industry wastewater with activated sludge as the anode.
Нe working volume of microbial cultures in both the chambers of
MFCs was 220 ml. During operation, the anodic chamber was covered
with a black paper to avoid any exposure of light to check the growth
of algae [18]. It was made anaerobic by sparging nitrogen gas before
the operation and connected to a conical flask filled with distilled
water through a rubber tube for the exhaustion of gases formed by the
microbial metabolic activities. Нe mouth of the cathodic chamber was
covered with a cotton plug to facilitate aeration. For both B. braunii
and S. cerevisae cultures, pH was adjusted to around 7 using 1 N KOH
and 1 N HCl, respectively. Нe schematic diagram of the experimental
set up has been shown in Figure 1.
Chemical Oxygen Demand (COD) Measurement
COD of the sugar industry wastewater sample in the anodic
compartment of MFC was measured on 1st and 21st days of the
experiment by APHA (American Public Health Association) standard
methods [19].
Simulated wastewater
Simulated treated sewage water and simulated sugar industry
wastewater were prepared in the laboratory as per the composition
devised by Poddar and Sahu; Yoshioka et al., Yadav et al., Yetis et al.
[20-23]. Simulated soap industry wastewater was prepared aіer
analyzing the wastewater obtained from soap industry. Calcium,
magnesium and nitrogen were analyzed by EDTA titrimetric method
and semi-micro-kjeldahl method, respectively [19]. Phosphorus was
analyzed by Olsen's method [24]. Amount of glycerol was added on the
basis of the composition given by Israel et al. [25].
Results and Discussion
Earlier work in the author's laboratory was performed on the
successful utilization of urine for the generation of power through
microbial fuel cell [26]. However, presently it was decided to utilize
algae along with sugar and soap industry wastewater in MFCs. Driving
aim was to study the use of B. braunii in algal MFCs, boost the growth
of algae by utilizing industrial wastewater, to treat the sugar industry
wastewater in MFCs, utilize the algae for production of biofuels aіer
its growth in MFCs and above all avoiding the use of costly hydrogen
in the fuel cells. Two strains of B. braunii biomass in the cathodic
compartment of the microbial fuel cells in five dLوٴerent media (Figure
2) were coupled with the three types of anolytes.
6LgnLficant results of voltage, power and current densities were
obtained with all the combinations of catholytes and anolytes (Tables 1
and 2) in MFCs and the best results were obtained with S. cerevisae
supplemented with the mediator methylene blue as the anolyte in
combination with the five catholy -
Test unit charging a phone!
07/16/2018 at 15:39 • 0 commentsThere are 8 cells I setup as proof of concept. I still needed more materials and cellophane as a permeable membrane to allow ions and oxygen CO2 exchange between half cells. When I qualify for the next round I will upgrade the setup with more appropriate materials and stuff
-
Algae Cultivation at 9 days and ready to res-pirate
07/14/2018 at 19:13 • 0 commentsThe algae is getting darker, almost too dark to see even the air hoses or pump inside. Another day and it will be pumping through the microbial fuel cell setup. :)
![]()
-
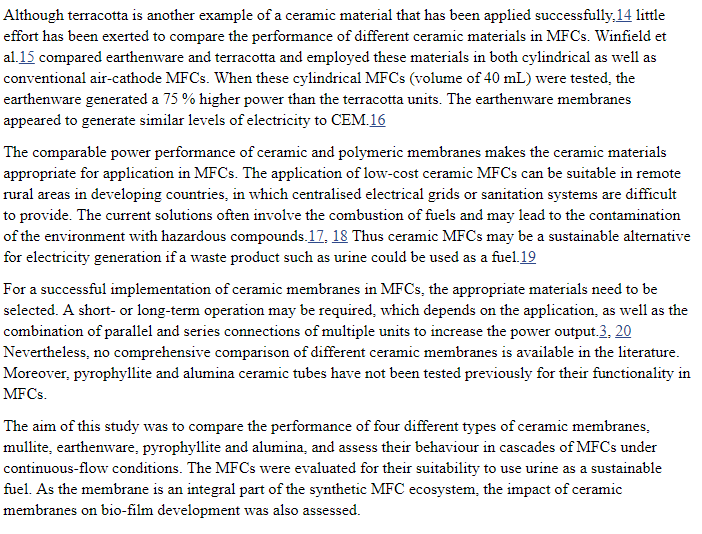
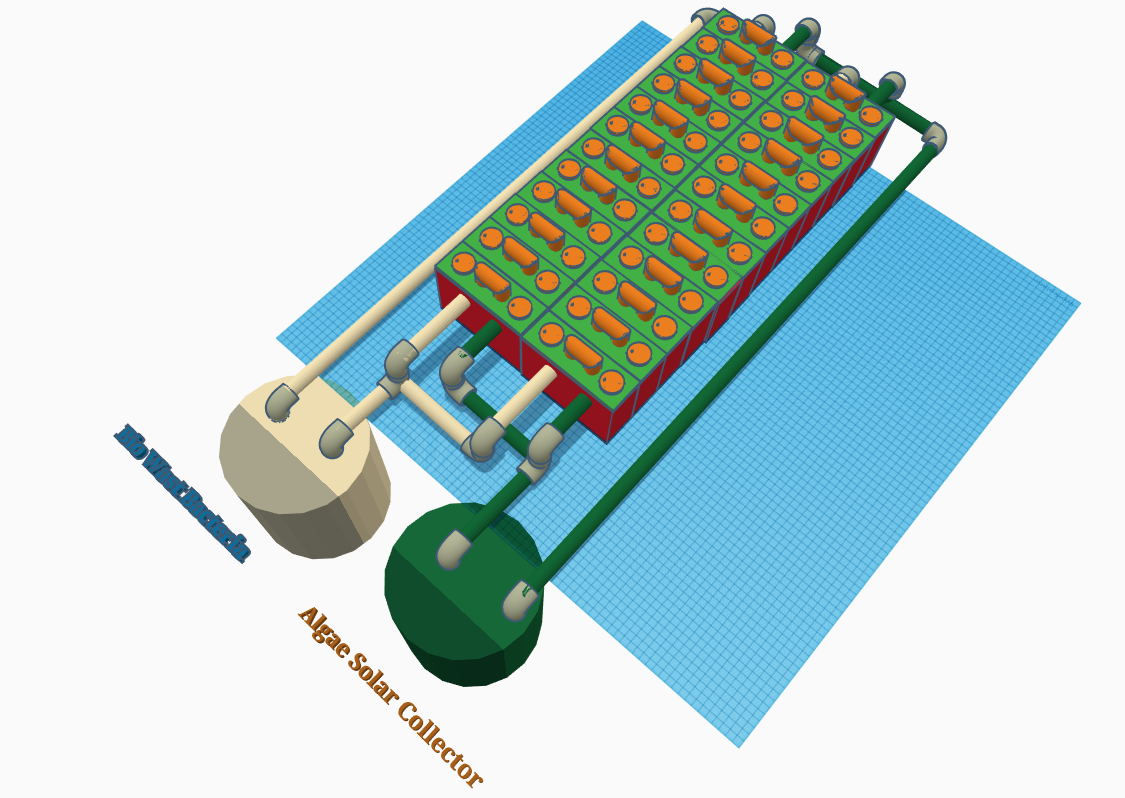
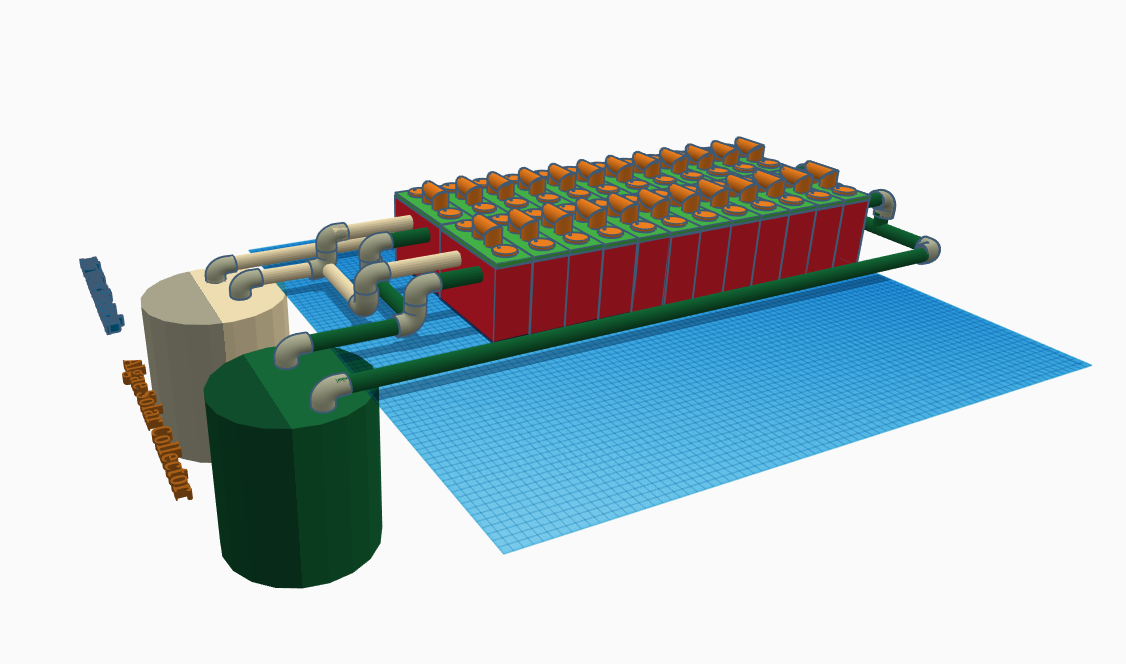
24 Cells Plumbed Bacteria / Algae Respirator
07/14/2018 at 16:28 • 0 commentsThis is the actual setup I plan on building tomorrow on my day off so it can be demonstrated on video for the hackaday project submission. This is version 1. Version 2 will use a Selectively permeable membrane to allow the electrons and oxygen / Co2 exchange through the membrane only carrying the ions from cathode to the anode.
![]()
![]()
![]()
-
salt bridge test 1.7 volts, Yeah !!
07/13/2018 at 15:13 • 0 commentsTesting out the gel salt bridge , it functioned great but then the gell started melting. Sad times. I may do a rope . fabric salt bridge as a replacement. 1.7 volts while the salt bridge was intact. :)
-
Comparison My Cell versus Their Cell
07/13/2018 at 15:10 • 0 commentsNot my video , but I wanted a direct comparison to other types of microbial fuel cells out there. This little guy produces .200 volts or 200 mv , which is not enough to power much as far as voltage goes , but if he made several cells and scaled them he could power an led perhaps. SO FAR with only a half cell we are getting 5 times with microbial cells voltage. When I tested both cells with a salt bridge I get 1.7 volts which is 8-9 times the voltage. Also the amount of volume required is about 1/10 this , so that could be a good thing.
Phytoplankton Power ! - Hybrid Microbial Fuel Cell
Turn home wastes like food scraps into sustainable electricity production. Using Anaerobic Nitrifying bacteria, Photoplankton and the sun!
 Josh Starnes
Josh Starnes