Triboelectric materials demonstrate a phenomenon that some give up electrons and some attract electrons when friction is present between them. Rubbing the two materials can present a static charge between the two. Similar to when you rub a balloon on your head and it will stick to a wall or cause your hair to stand on end. This phenomenon can be used to generate useful power. As the materials are seperated an electrical potential is present.
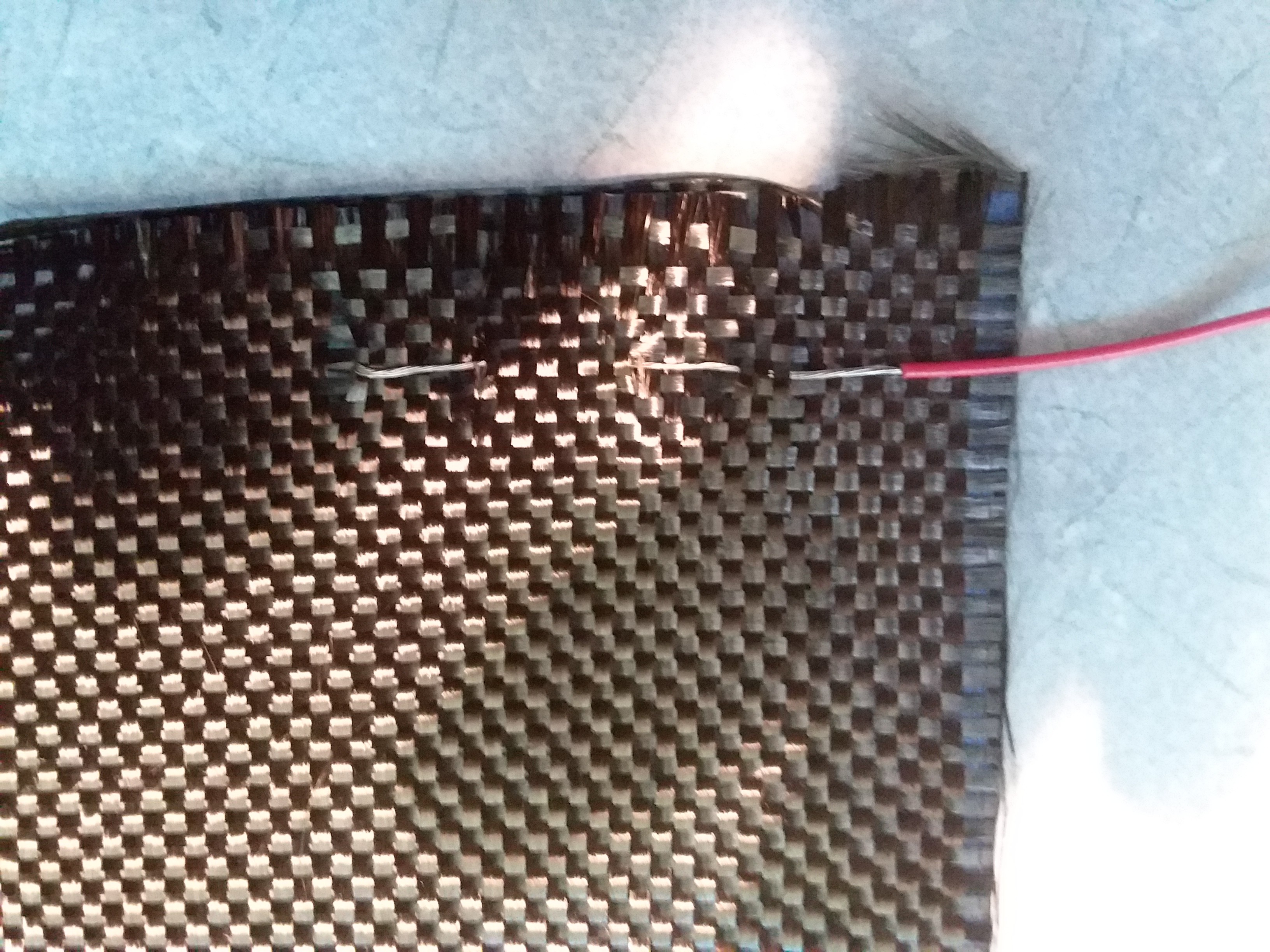
I noticed a few months back while using epoxy filled with diatomaceous earth to thicken it that it, when cured, had a strong electrostatic attraction to the nitrile gloves I was using. I looked further on the internet and saw there is a new class of materials called triboelectric nano-generators. The proposed theory for the efficiency is that materials with nanoscopic details present more surface area for the momentary bonding and electron exchange between them.
Looking at a table of triboelectric materials we see that silicone (and silica the primary constituent of diatoms) is very negative on the spectrum and natural latex rubber is very positive. Diatoms and fused silica have very fine nanoscopic structures that should satisfy the criteria for higher efficiency. Here I go again making a mockery of legitimate scientific research.
I decided to throw something together the last day of the challenge as there is always that hackaday post by Brian urging the community to do so.
I could claim all sorts of savior of the third world savior self proclamations, or charge your cell phone by walking but all I have is a proof of concept and no oscilloscope to determine output. I can light some LED's in the dark but I will continue this work further as I have pretty much perfected my version of low cost DIY supercap/batteries and should be able to roughly quantify output at a later date while using the former to store energy produced.
Maybe I can come up with an implementation that allows the use of these materials to help in areas where electrical power is not easy to come by.
 MECHANICUS
MECHANICUS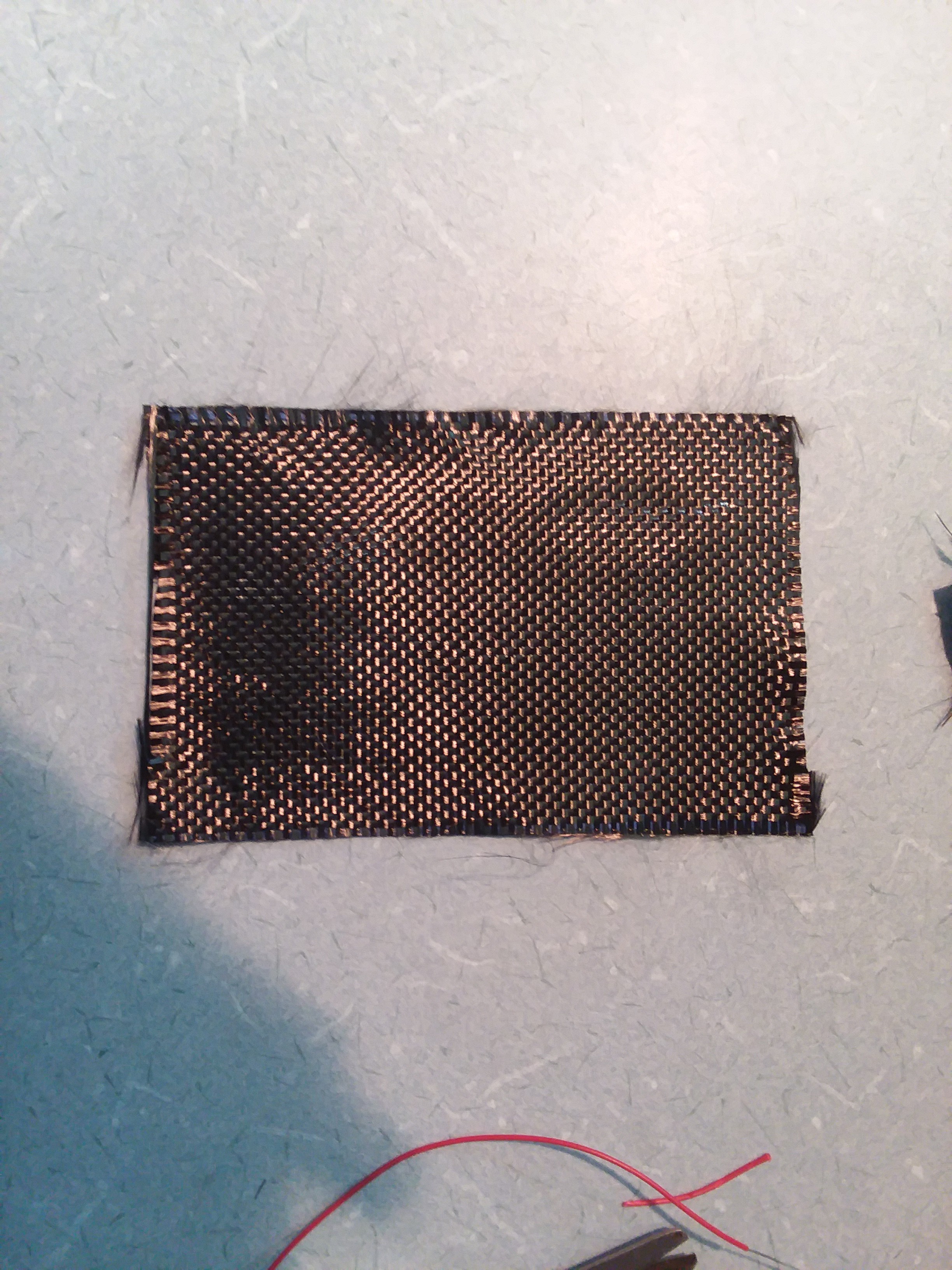

 atelierlelu
atelierlelu
 Giovanni Carrera
Giovanni Carrera
 G. Rosa
G. Rosa
 Wasim Sahu
Wasim Sahu