You can find our previous hackaday page for season 2 kit versions here.
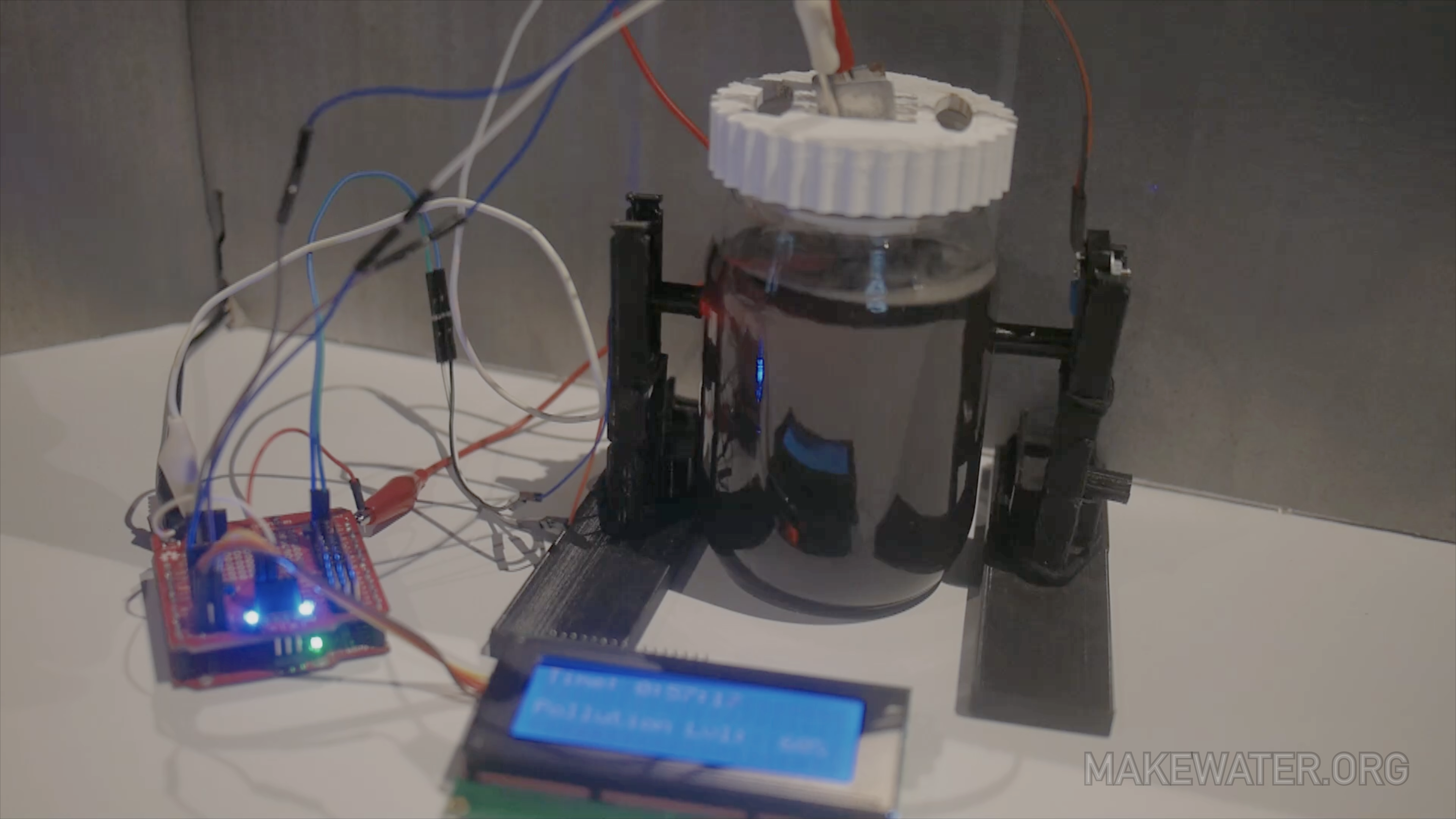
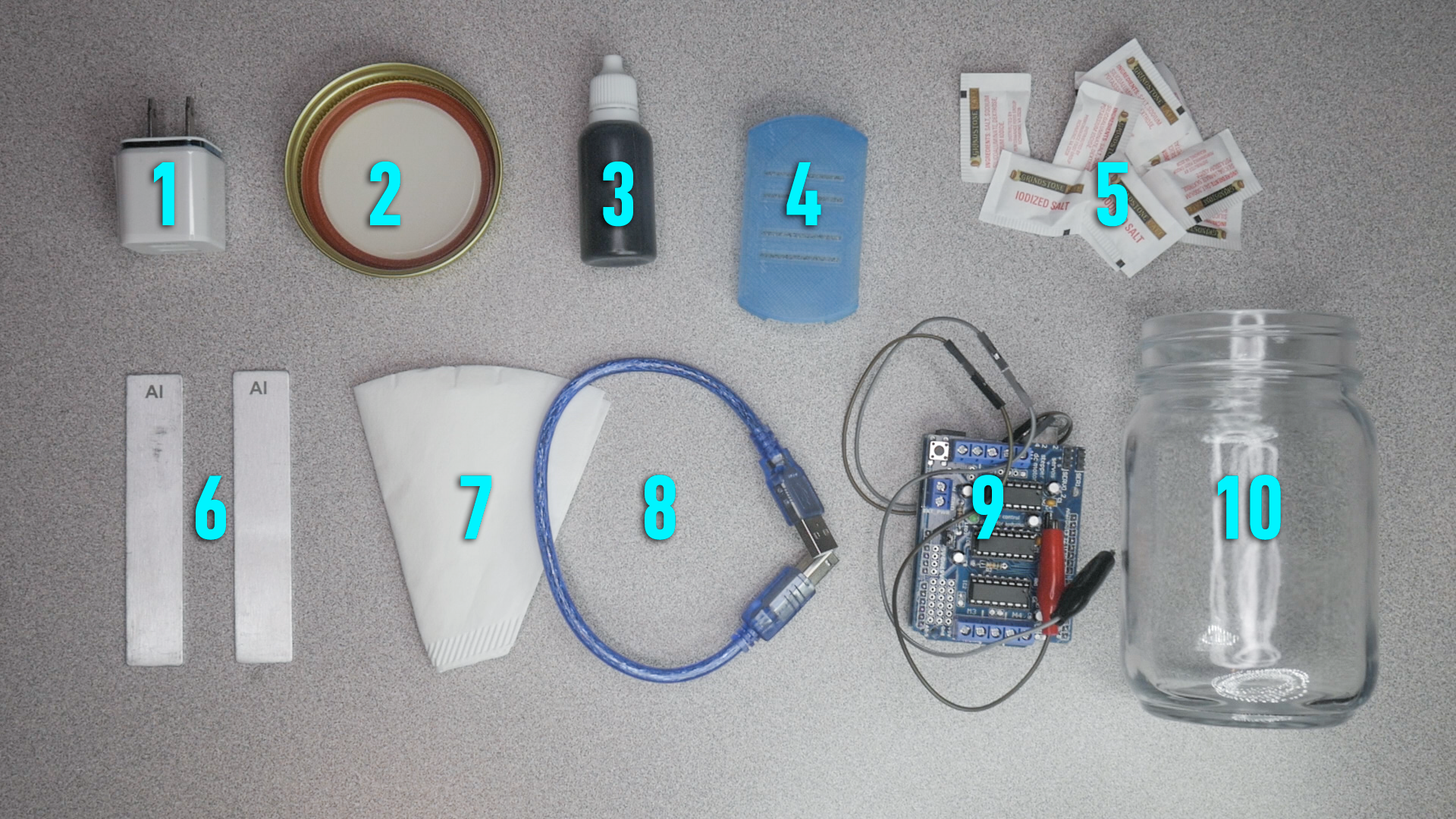
Coagulator Kit & the electro-coagulation process
Electrodes are submerged in a container of ‘dirty’ water. Electricity flows through an electrode and through the water to the other electrode. This completes the electrical circuit. As electricity passes through the electrodes, metal ions are released into the water. On the surface of the electrode, water is split into hydrogen gas (H2) and hydroxyl groups (OH-). When electricity flows through the water towards the other electrode, surface charges on suspended solids are destabilized. This reaction causes suspended solids, metals, emulsified oils, and other contaminants to clump together, forming what is called flocculent. As electricity flows metal ions (Me+) are released from the electrode and attach to the flocculent. Because the electrode is slowly losing metal ions, it is also called the sacrificial electrode. The flocculent may either float to the surface or sink to the bottom depending on the density and structure of the contaminants.
Electro-coagulation can change other chemical properties of water (such as pH, alkalinity, and hardness) and make it taste better. Contaminants such as bacteria and viruses may also be immobilized or killed, which then can be filtered out of the water with the coagulated solids. However, electro-coagulation is considered a pre-treatment process only. To ensure the water is free from bacteria and viruses, additional treatment is required. While the concept of using electricity to treat water is not new, recent technology in electronics is making electro-coagulation less expensive, more accessible, and more efficient in filtering water. This makes it possible for a greater number of people to have cleaner drinking water.
Safety:
Always stay cautious and aware of surroundings when operating the coagulator kit. There is a small current that runs through the electrodes when in operation. As soon as the usb is plugged in the current will run through the alligator clips. Although it is a small current do not touch the exposed metal alligator clips and/or the electrodes once the power is on. Keep in mind that hydrogen is created which is combustive so do not pull clips off electrode while in operation this could cause a spark. Also note that small amounts of chlorine are produced when using salt in the mixture.
When operating the kits please do so in a large well ventilated room.
 Ryan Beltrán
Ryan Beltrán




 pandoras9
pandoras9
 Bart.remans
Bart.remans
 MakeWater
MakeWater