LINKS SHORTCUTS!
⚠️ PolySense got the wildcard award of the Hack A Day prize!
| 💥 MORE NEWS! 💥 1) 🎓 RESEARCH 2) 💀 HACK A DAY ARTICLE 3) 🤖 MAKER FAIRE 4) ⚡ ARS ELECTRONICA | 📜 PROJECT LOGS📜 1) 🔬 REVERSE ENGINEERING 2) 🥽 HCI EXPLORATION 3) ✨ ART INSTALLATIONS 4) 🔥 HEATING + SENSING |
Application examples



What!?
We use a chemical process called in-situ polymerization (explained later).
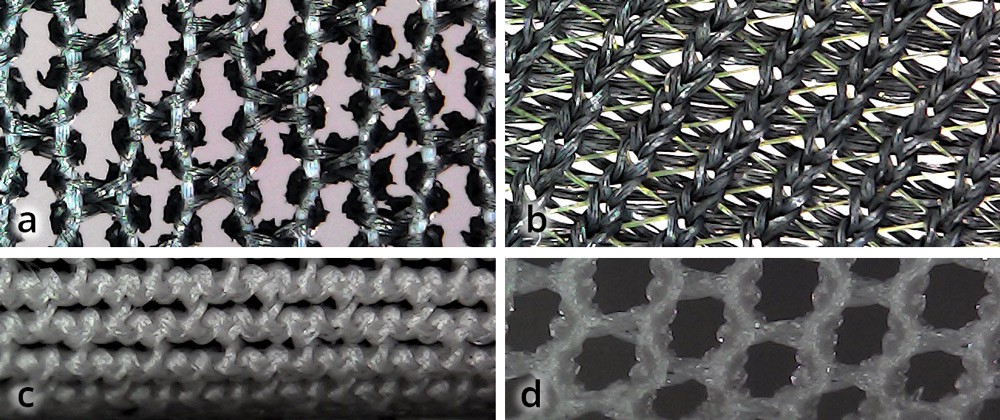
It allows functionalizing almost anything fibrous and porous materials (natural ones like cotton or cork work better than synthetics in general).
Once polymerized, your originally non-functional material ends up with sensing capabilities:
- pressure
- stretch
- capacitive
- humidity
- temperature (bonus: heating is also possible)
Why!?
In our hackerspaces and research labs, we explored musical textile interfaces and we used a commercial piezo-resistive material (pressure sensitive).
The only good one was expensive and became hard to get because of a new exclusive contract with another company.
So with material scientists, we reverse-engineered it, and made a DIY process simple enough for the kitchen of our hackerspaces.
Illustration of the piezo-resistive effect:
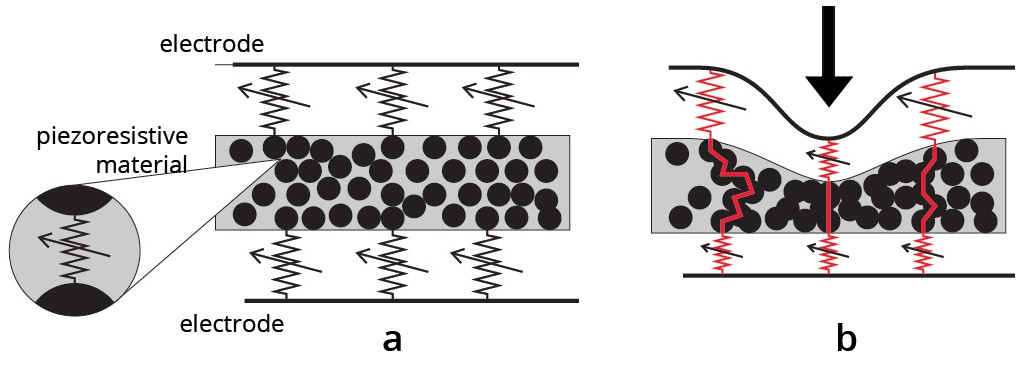
How?
The following video summarizes the process, but you'll need chemical products:
- Europe source: Pyrrole + Iron Chloride
- US source: Pyrrole + Iron Chloride
...and get a machine to mix your materials for about 1h, example:
- chemistry magnetic stirrer
- ice cream maker
- a batter mixer
- a camping washing machine
- or you can build it with a drill and a bucket for example.
Protocol summary
(adapt X to your quantity):
1) Water: ( X ) ml - fill the container so that there's about 75% of textile (but don't put it yet!)
2) Pyrrole: ( X / 250 ) ml - add it and stir it
3) Material: add it and keep stirring for 10 minutes
4) Iron chloride: ( X / 100 ) g - keep stirring for 30-60 minutes depending on the material
5) for capacitive sensors: you can repeat this procedure, or multiply these proportions and polymerization time by 2 to 5 (depending on the material too).
💡💡💡💡💡💡💡💡💡💡💡💡💡💡
The process, called in-situ polymerization, is particularly unique because:
- First we soak the textile with the monomer (steps 1 to 3)
- Then we trigger the polymerization (in-situ, or in place)
This reaction creates "a kind of molecular dyeing with carbon" which has much stronger properties than a coating approach.
💡💡💡💡💡💡💡💡💡💡💡💡💡💡
Sourcing the products
The chemical products were ordered from 2 possible sources:
- Europe:
https://www.glentham.com/en/products/product/GK9750
https://www.glentham.com/en/products/product/GK2873
- US:
http://fishersci.com/shop/products/pyrrole-tci-america-3/P057425ML
http://fishersci.com/shop/products/iron-iii-chloride-hexahydrate-99-analysis-acros-organics-3/AC217091000
Note: every material reacts differently so you'll have to do a couple of tests to get the right chemistry ratios, and the right timings...
 Cedric Honnet
Cedric Honnet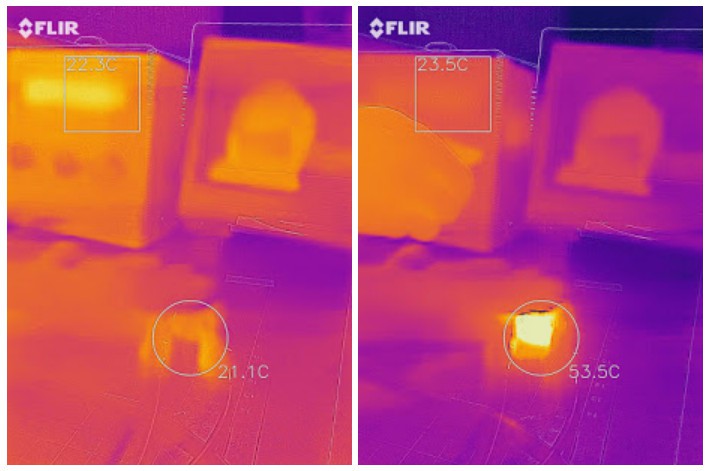
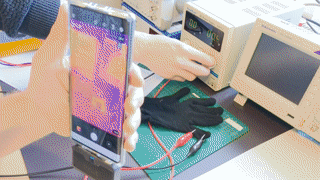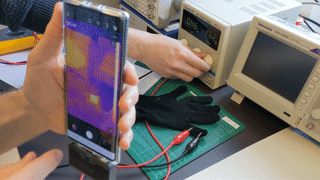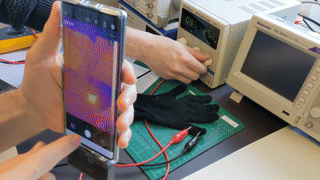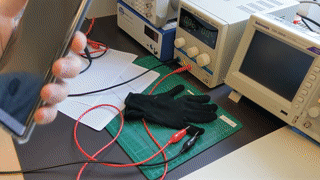
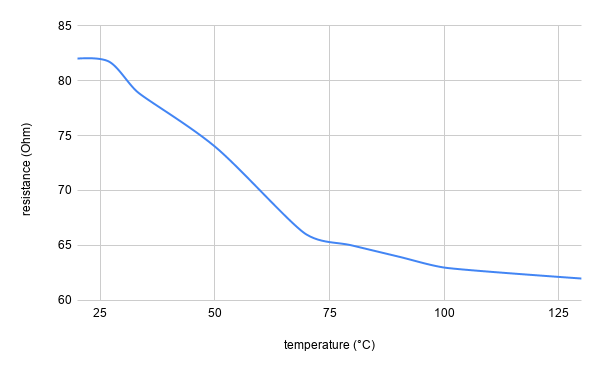



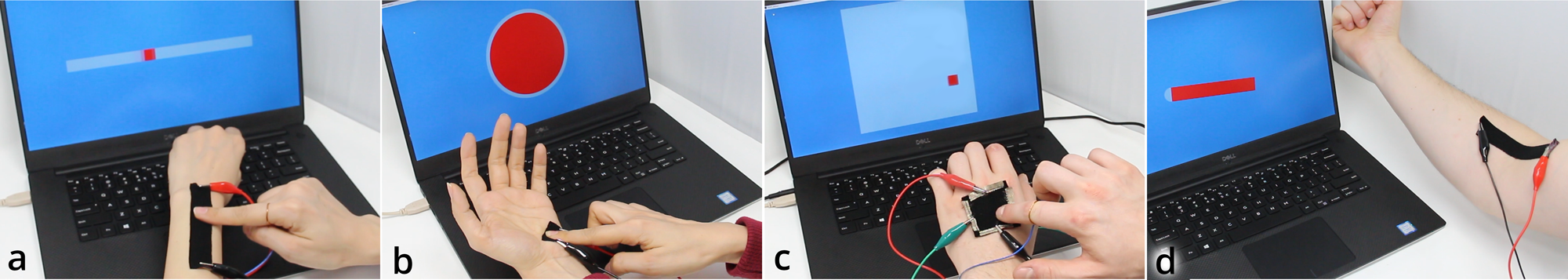 Note: an academic paper about it was published at the
Note: an academic paper about it was published at the 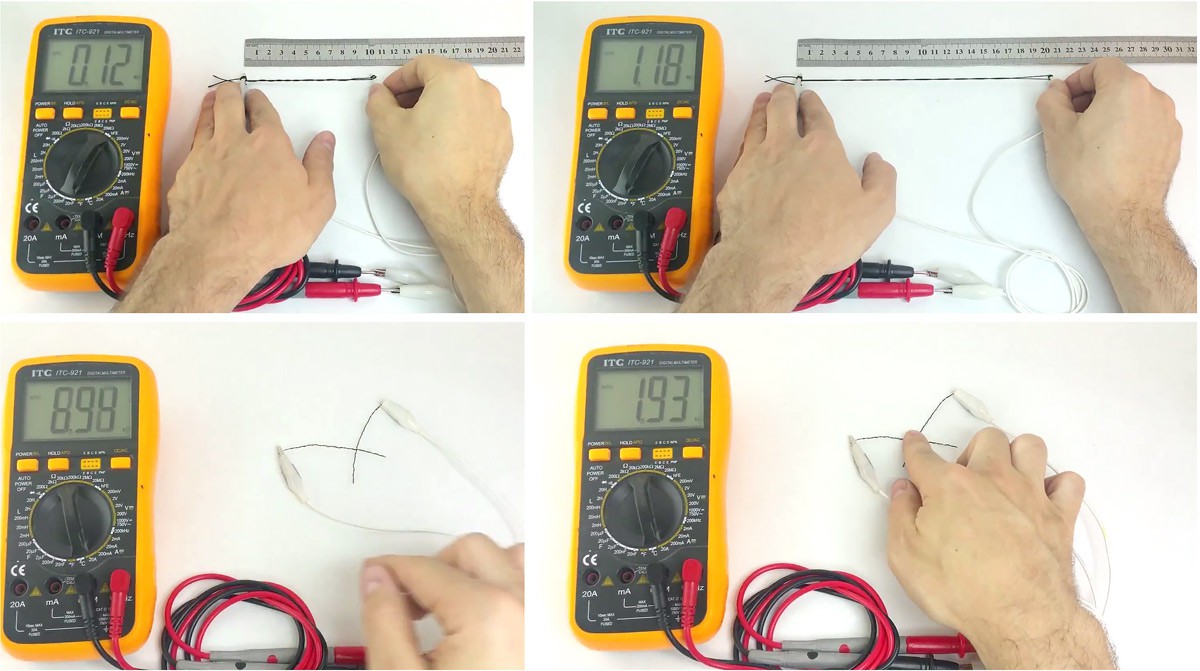











 Chuck Glasser
Chuck Glasser
 Boris van Galvin
Boris van Galvin
 xdylanm
xdylanm
 Michael Barton-Sweeney
Michael Barton-Sweeney
Thanks for your great documentation!
that is really an interessting e-textil-concept and i like to try it!
in your yt video you are using a *camping washing mashine* ?