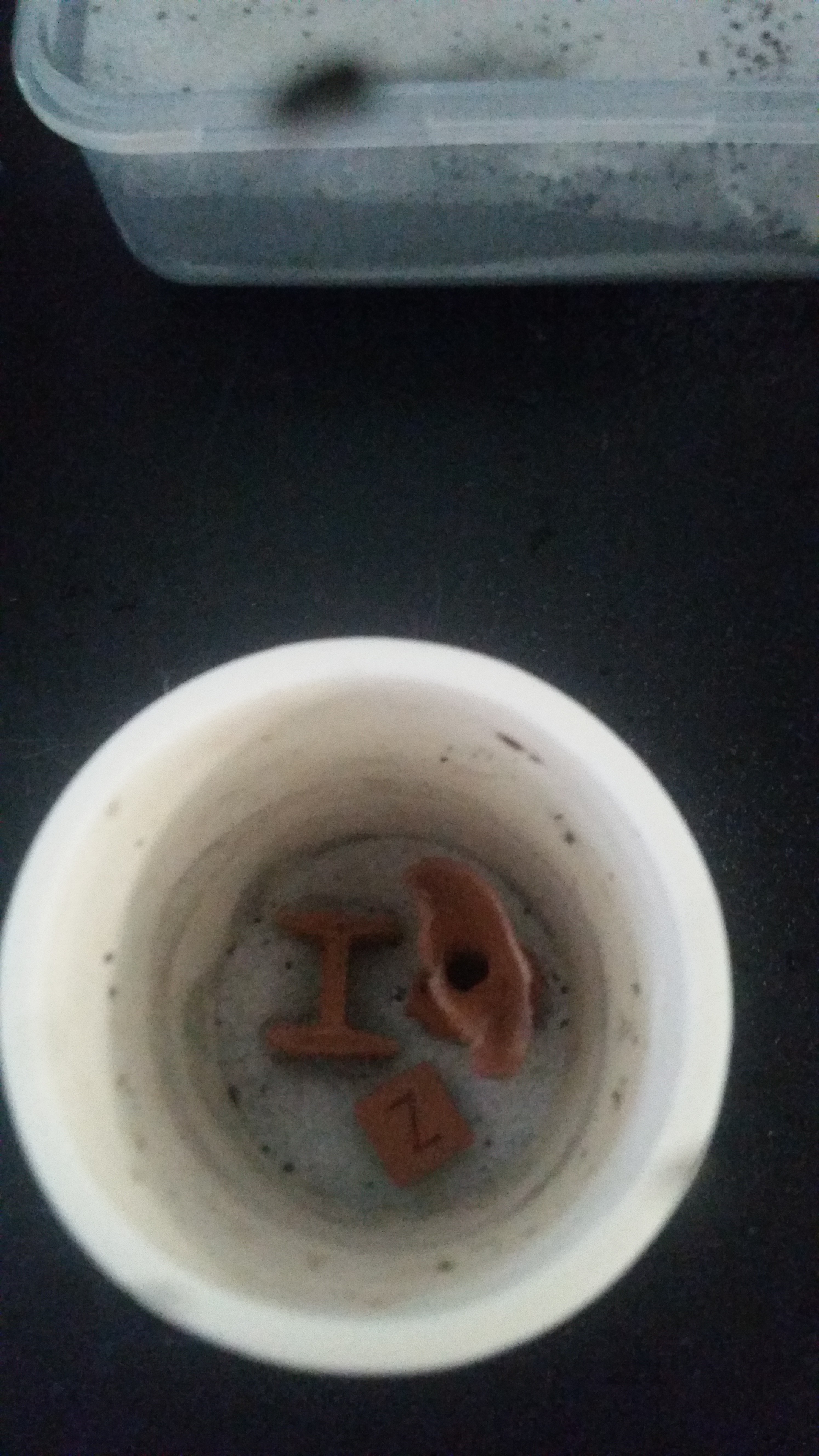-
Bound goop deposition at (lab)
10/11/2021 at 20:05 • 0 commentsFirst real quick, here's a video showing some of the progress I made with printing copper:
But now I'm afraid to say this might be my last post on the subject, because I'm deviating pretty far from the "metal" aspect of the original project title. And that's fine. Goals change. I thought it was really cool to do glass, for instance. Now I'm pretty involved in printing goop.
Hydrogel goop:
![]()
Honey-alginate goop (this is supposed to be my head):
![]()
Toothpaste goop:
![]()
Wait, toothpaste goop? Apparently, yes, it prints surprisingly well! Better than honey or caramel or even the commercial hydrogel, anyway. It's not great, but go slow and you can make shapes that actually survive with high fidelity after drying out.
I've been trying to be at least somewhat scientific with this, for instance, using this fancy machine to measure the rheological properties of my honey:
![]()
![]()
What's interesting is that all this machine does is measure resistance to applying torque, which means you can do something very similar by measuring the power requirements for a handheld drill stirring a liquid. (I learned this from Brad Woods when I went to the virtual foundry). The main difference is precision which is why this machine costs $120,000...
And basically from these results we can see that honey indeed undergoes shear thinning, and in theory this means you can 3D print with it if you get its base viscosity high enough. In practice, getting that viscosity high enough without using additives means that it's an emulsion of liquid and tiny honey crystals. And when you try to force it through a small nozzle, it separates and clogs. Lot of work to do there.
But anyway, I'll close this off with a video of my true success, and in my opinion a crowning achievement (ha!) of my life: Science toothpaste. -
Bound glass deposition printing at (lab)
07/20/2021 at 22:34 • 5 commentsSoooo... this is not metal. But it is performed in almost exactly the same way as metal.
Last month I visited the virtual foundry, the company that produces these metal filaments. And while I was there, I helped extrude a new kind of filament: Glass filled.
And it has quickly become one of my favorites. It just has a lot of interesting properties: It’s lighter than PLA, has a high fill but is still flexible and very strong, looks cool, and unlike copper can be sintered overnight in one step (glass is already bound to oxygen, so you don't have to worry about oxygen).
Here's what comes out of the printer:
![PXL_20210629_205316198]()
![PXL_20210711_214627539]()
Check out that sparkle. When it prints, it does so super smooth with great detail! Clogs are a frequent issue, though on my creator pro with 0.4 mm steel nozzle. I even upgraded to an all metal hot end to try and fix it, which turned out not to help much. What did help, was lowering the temperature from 220 to 200 or less. I think that the plastic and glass has a tendency to separate when left sitting in the nozzle, and a lower temperature helps somewhat. I have properly taken off my nozzle and removed a solid plug of material at least a dozen times though.
![PXL_20210629_211412340]()
My computer is broke so I can't take a proper scan of it, but I would like to (before and after sintering), because they look really cool.
Debinding and sintering trials: it actually only took two tries to get something to work. Units in Celsius:
Ramp to 0-204 over 30 minutes
Hold at 204 for 2 hours
Ramp to 482 over 2.5 hours
Hold at 482 for 3 hours
Let cool for 2 hours (possibly unnecessary)
Ramp to sintering temperature over 4 hours
Hold at sintering temperature for 2 hours
Sintering temps tried so far (first based on this paper). 700, 800, 900, 1000, 1100 Celsius.
Results:
700 just falls apart when you take it out.
800, 900, and 1000 are white and porous, with decreasing porosity and increasing strength:
![PXL_20210710_230645420]()
![PXL_20210711_202038803 (1)]()
You can see that the expansion during debinding is not yet a solved problem:
![PXL_20210710_233636078]()
But note that there IS isotropic shrink, which was totally unexpected to me — my understanding of sintering involves production of vapor (thanks to a prof who schooled me about it), which I didn’t think glass would do. It also isn’t fair to say that it “melts” at these temperature, but rather just softens. Either way, binder goes bye-bye and glass gets stuck together.
Here is a video (proper hackaday --- hint hint, tip line) where I use the porosity to make coffee:
I tried it so you don't have to!
One problem is that the aluminum oxide refractory gets fused to the surface of the glass:
![PXL_20210710_224830107]()
This is a problem because uhhh this is a problem. Especially for eventually making transparent pieces. So I’ve been experimenting with using magnesium sulfate as refractory material instead of aluminum oxide, with the idea that, even if it gets stuck, it would dissolve in water afterwards.
This worked but it shrank and became like plaster, being a pain to get out afterwards.
![PXL_20210715_185035604]()
You can see the comparison though, in aluminum oxide on the left and mgso4 on the right. No sticking!
![PXL_20210715_193803352]()
Next I want to take the temperature past 1100, the part where it starts to become solid glass, with the goal of transparency. I think this is possible to some degree --- there's a company that does this using resin, with nanoparticles of glass (the ones in here are a bit bigger, maybe ~50-100 ish microns across). So far it just blobs up. The pieces make that nice glass sound, though.
-
Everything on my first roll of copper
06/23/2021 at 19:57 • 0 commentsSo I've done a lot more since the last time I updated. Completed my first roll of copper! Here's almost everything left:
I've also tried a couple more materials, and did experiments like with debinding:
or washing:
Or tensile testing:
Most of the discussion for this, however, has been localized to a forum specifically for this filament. It's still experimentation but maybe not quite "hackaday". But if you're interested, you can check out more of my musings over at https://discourse.thevirtualfoundry.com/. -
5, 6, 7, 8! Heat makes metal congregate!
04/16/2021 at 22:29 • 3 commentsHere's a quick pick of all of these, plus some earlier trials:
As we can see, we have had some success! Let's go through this, shall we:
Attempt #5:
Leave uncovered for first step, with some carbon on top.
30 mins to 204. Hold for 2 hours. 2.5 hours to 484. Hold for 3 hours. Let cool down.Add more carbon. Cover.
5.5 hours to 1018. 2 hours to 1074. Hold for 3 hours.
VIOLA!
![]()
Metal parts! The cube, the I beam, and the "head". Totally solid, with a smooth shiny texture, and a nice clink when you drop them! Progress!
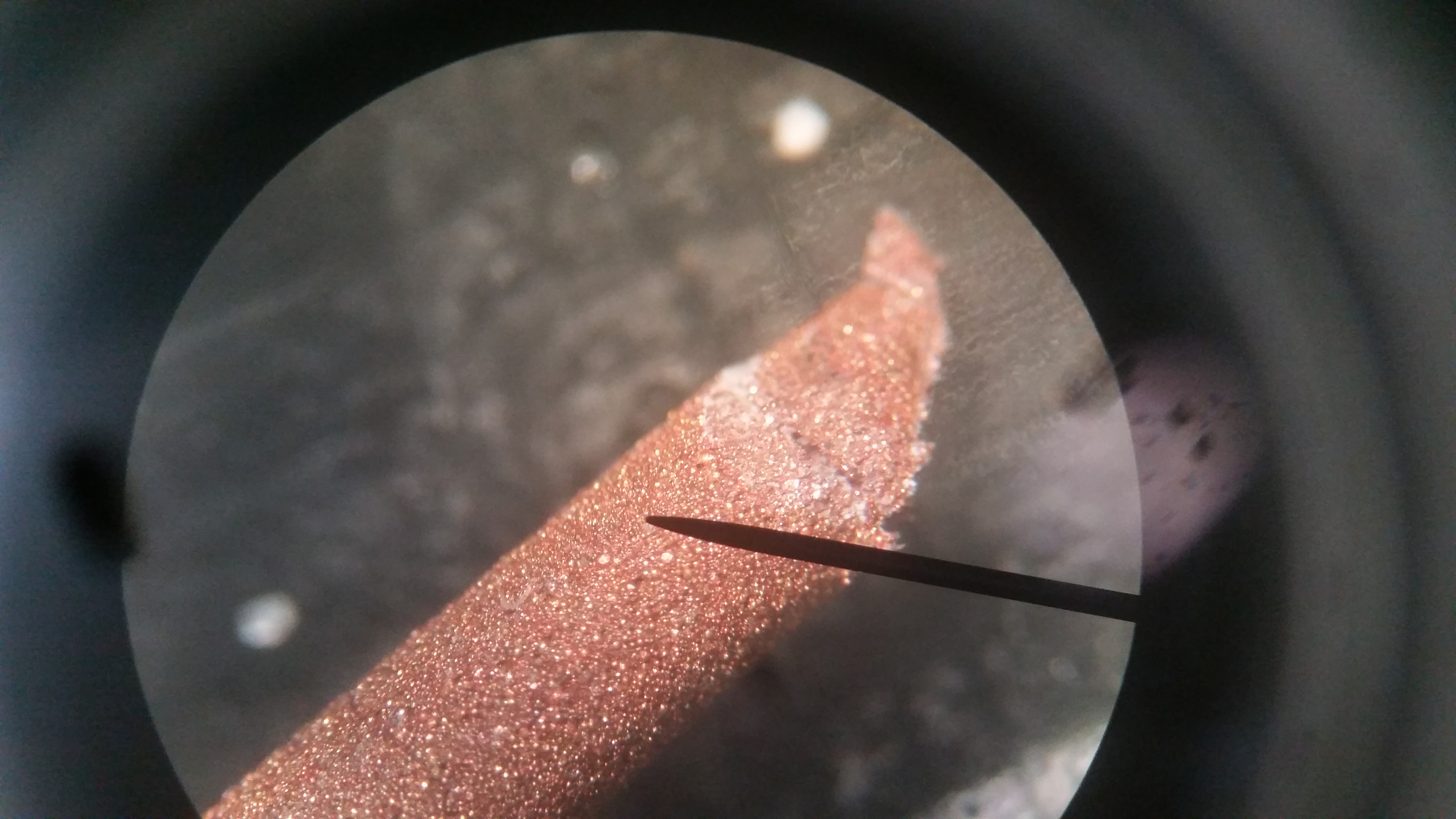
They look like this close up:
![]()
I'm sure you know of the LadyBug Beefy scanning project if you know of this one, but if you don't, check it out. I use a digital microscope and a CNC-type setup to take lots of pictures and stitch them together.
![]()
We can see just how much this has shrunk. (a lot). The letters on one or two sides are just barely visible, while the Z axis one is completely gone. But it's roughly flat on the bottom, so some shape has been preserved. The I beams faired even better but definitely still with drooping for the part that wasn't being supported by the aluminum oxide refractory.
At the same time the density and strength for these is great. I would be totally happy if I could get this density and strength for a functional part. We're not quite there yet though, as you will see.
Attempt #6
Same temperature profile as attempt #5, but in one cycle adding carbon beforehand, uncovered, in a much small and fancier oven. Result:
![]()
Nothing was left. Not even an oxidized part like #1. Carbon was completely gone, too. I'll investigate this oven later, since it's high quality, but for now I'll stick to my current oven.
#7:
Leave uncovered for first step, with some carbon on top.
30 mins to 204. Hold for 2 hours. 2.5 hours to 484. Hold for 3 hours. Let cool down.
Add more carbon. Cover.
5.5 hours to 1018. 2 hours to 1060 (14 less than #5). Hold for 3 hours.
So I ran the second cycle for this one and I actually let it ran twice by accident. It got about an hour through the second sintering hold at 1060 before I stopped it. Turns out "cycle 2" does not mean it can store more than one cycle, but rather, it just does it twice. Whoops.
And yet this one turned out to be the best yet in terms of holding features!
![]()
Check it out! It's my head! In solid copper! I mean, it looks like a little shrunk, but that makes sense because on one side it was hollow (weird scan). So yeah! Woot!
I really should have measured all dimensions before making this post, whoops, and I will once the process is more stable. But look at that I beam! And the cube! Almost every feature was preserved --- marks from pulling it off the build plate, overhung strings, and so on. This would make some great miniatures.
My only gripe was that the density was clearly not maximum un, for instance, the I beam. And I want that. For functional parts, it is going to be necessary. And so I reran at just higher temp to try and reach that sweet spot:
#8
Leave uncovered for first step, with some carbon on top.
30 mins to 204. Hold for 2 hours. 2.5 hours to 484. Hold for 3 hours. Let cool down.
Add more carbon. Cover.
5.5 hours to 1018. 2 hours to 1064 (4 more than #7). Hold for 3 hours.
This one was intermediate, but unfortunately, not just "better":
Before printing:
![]()
After sintering, before cleaning with HCl ( have to make a video of that --- it's like magic, really)
![]()
![]()
Monsters! Eek! No face but in kind of head shape is really scary :(
It's definitely intermediate... so how to prevent it from sagging? One option is maybe to use that lower temperature but to raise the holding time. Another is to hold for a few hours at the lower temperature, then slowly raise to the higher temperature after it's had time to settle. But there's already such a long raise temperature time... I'm not sure. I'll have to try.
But I'd consider this a success. Let's see if we can get some more!
Update: We got some more! -
#4
04/07/2021 at 19:01 • 0 commentsChange: Increased sintering temperature to 1150 (from 1100), increase presintering temperature to 1040 (from 1030).
This goes in:
![]()
This comes out:
![]()
So yeah. Not that great.
Interestingly enough, each bump up of temperature resulted in less shiny metal existing in the final product. I would have thought that, even if sintering didn't go correctly, it would have at least resulted in a puddle of metal at the end. But no. Hmm...
I did notice that my carbon at the end (reused) was more metallic slag than carbon, so maybe I shouldn't be reusing that. But I don't think that was the main problem.
I have a theory that my covering the crucible and doing the whole thing in one step is interfering with the thermal binder removal. After all, in the few commercial settings that choose to use thermal debinding (as opposed to solvent or catalytic removal in mist or liquid), they use a vacuum. Know what the opposite of a vaccum is? A sealed container! It's not quite truly sealed, but maybe it's interfering somehow...
So for my next attempt, I'm going back to attempt #1 with the exact original recommended temperature and holding settings and doing it in two steps again. However, this time I'm adding carbon in the first step where it's uncovered (can't hurt) and then adding more carbon and covering it in the sintering step. After all, I ended up with a cube, I just want it to be less oxidized... right?
-
Attempt #3
04/05/2021 at 20:27 • 0 comments![]()
![]()
![]()
GRR!
The only change I made this time was that I bumped up the temperature from 1018 to 1030 (for the presintering step) and from 1074 to 1100 (for the sintering step). Was hoping this would counteract the extra insulation a bit. No dice.
I am a bit apprehensive to just increase the temperature even more --- see the melting in the I beam? That can't be good! But I'm not really sure what else to do otherwise. Still no oxidation, which is good, but my parts aren't solid! The cube is even worse than before, even.
maybe I can extend the ramping time from presintering to sintering temperature? Like for 4 hours instead of 2.5 hours?
-
Attempt #2: Insulation reduces oxidation, decreases peak temperature
04/03/2021 at 17:10 • 1 commentFew days ago my first attempt ended all crusty. There was some metal inside, so sintering did happen, but it was all oxidized and full of holes. The protective layer of carbon was all used up on top, too. Hmm....
I printed a few more doohickeys including (part) of a coin that I had scanned with my LadyBug project:
![]()
![]()
As you can see, it failed part way through. So two main issues with this process --- the printing process, and the sintering process. Rather than filament these things should be like 3-5mm straight rods that get pushed down. But this is what we got right now, and I am frequently fighting with jams.
Once I had my parts I changed a few things about the sintering process. One is that I measured my carbon: About 50 ccs.
![]()
I'm also using a thicker /bigger flask to accommodate more carbon, which I didn't think would make a difference otherwise, but it turns out to be one of the important details,
And I'm using this "refractory sheet" (fancy ceramic tile) that I found in the supplies to cover the crucible while it's in the oven. The goal of this was just to limit oxidation. Very, very simple idea, and one that turned out to be critical.
Finally, I changed the actual sintering settings. Instead of doing the debinding first and then sintering after everything cools, I'm just doing them both in one step. I also held the debinding and sintering temperatures for an extra hour, making the final settings:
Ramp to 204C over 30 minutes (changed from 10 minutes)
Hold at 204 for 2 hours.
Ramp to 482C over 2.5 hours.
Hold at 482 for 4 hours. (changed from 3 hours)
Ramp to 1018C over 5 hours.
Ramp to 1074C over 2 hours.
Hold at 1074C for 4 hours. (changed from 3 hours)
That's a long process! 20 hours. I came back the next day and found it not quite yet cooled. Rather than force it, I just waited an extra day.
When I came in, there was a very good sign: Unused black carbon.
Actually, the flasks were full of it --- only the top few millimeters consumed. This despite the fact that I added the carbon before debinding this time and held at temperature longer. Conclusion: Covering the crucible makes a huge difference in terms of how much oxygen gets in there. Good to know!
Digging out my parts, they looked quite different from last time.
Roughly holding their shape, still pretty heavy, but black and with bright copper protrusions every so often.
Oh, and the non-copper bits behave like this.
Yeah, that's not supposed to happen. Every part easily crushed like this to some degree, though --- the cube still had some structure, but the LTU logos for instance turned to powder. And yet, sintering was happening, and the resulting copper was both brighter and not brittle at all.
Throwing all this into some hydrochloric acid again, I observed only a very small reaction (which would indicate the presence of oxidized copper dissolving). Again, just a quick comparison, here's my part after soaking in HCl last time:
![]()
And this time:
What this means to me is that rather than being sintered and then oxidized (like last time), this time my parts are mostly still "brown": Binder removed, not yet sintered. This makes sense, because my thicker flask and refractory sheet are probably acting as good insulation --- the inside of the flask is not reaching the critical peak temperature!And that critical temperature actually has to be very close to the melting point of copper --- the reference melting point is 1084 C and I was setting the oven to 1074. This is compared to the sintering temperature of cold compressed copper powder at 750-1000C. Rather than sintering, the industry might call this "liquid metal diffusion".
And, incidentally, the industry totally does this all the time in the form of "metal injection molding", which is super cool and actually the best way to make small, complicated metal parts. They just squirt the metal and organic binder into a preformed mold and then also toss it into an oven. This is very helpful for my research since the process is much more documented than "metal sintered 3D printing". And it gives me higher hopes for the final part quality, since it's used at scale for tons of purposes. Parts made in this method from wikipedia:
![]()
So, yeah. Trying again, this time with a bit higher temperature in the sintering step. Biting my nails figuring out how much higher. I figure since I've gotten some metal, it doesn't have to be that much more --- 20C? 30? 50?
-
Attempt 1: A head and a cube
03/30/2021 at 18:12 • 0 commentsFirst challenge: Printing with this filament. It's not the easiest to work with.
![]()
(PS: Sorry for all the black goop on the pictures. My phone camera protective glass broke off and these are a permanent feature now.)
Here's what it looks like under magnification:
![]()
So yeah, it's basically just PLA mixed with copper particles, as far as I'm aware.
it's pretty brittle and they recommend using this thing called a "filawarmer" to soften it before it goes into the hotend. I tried it and found it to be more trouble than it was worth. My general strategy has been to just alleviate strain and loops as much as possible, and that's worked okay.
First I tried using a Lulzbot since they recommend using a 0.6 mm steel nozzle, and it has that. The 20 minute long startup sequence made it impossible to iterate, though, and so I switched to a creator pro which I haphazardly installed the steel nozzle from an all-metal hotend upgrade but without using the steel throat, on account of I didn't want to. This meant snipping the PTFE tube approximately to the right length. It also meant using a 0.4 mm nozzle --- smaller than they recommend --- but I figured that if I couldn't print at that feature size then metal printing was pointless for my purposes anyway.
Then you gotta position the filament using the OSHA recommended method of strain relief:
![]()
That thing is never gonna fall down!
![]()
oops.
Okay, let's try printing stuff. They recommend using standard PLA settings but no retraction and a VERY slow print speed (20 mm/s or so --- one reason is the metal has a high thermal mass, and also I'm not using fan cooling). Also, solid parts --- I'll try infill at some point, but this alone really makes you want to print small things!
I also found that getting the level right was critical. I use the extremely scientific method of placing an increasing number of sticky notes under my limit switch until it works.
![]()
Boom! prints! it actually is not that bad for producing just the "green" parts once you get the settings figured out. And wow, do they feel heavy in your hand! Way more than you'd expect a little 1 cm cube to be. I will probably keep a couple of these around just for that shock value.
Next: the actual sintering process. The basic procedure is pretty simple:
1: Place your parts in your crucible and surround them with aluminum oxide. This acts as a support material when the binder (PLA) is removed.
![]()
![]()
![]()
Now you can do step 1: binder removal. The procedure I followed was:
Ramp to 204C over 10 minutes.
Hold at 204 for 2 hours.
Ramp to 482C over 2.5 hours.
Hold at 482 for 3 hours.
Next day I came by, pulled the crucible out, and dumped carbon on top. I then sintered with the following conditions:
Ramp to 482C over 20 minutes.
Ramp to 1018C over 5 hours.
Ramp to 1074C over 2 hours.
Hold at 1074C for 3 hours.
(Next time I am going to do both debind and sinter in one step with more carbon to save time. )
![]()
Hey, that looks pretty good! You can even still see my face! except... nothing has been shrunk. Which is weird. Some kind of crust, maybe?
Turns out it does in fact peel off in some areas:
![]()
I also tried using ultrasound to clean them with no dice.
What did work was good ol' hydrochloric acid. Dissolves copper oxide, you know?
![]()
![]()
(rotated to the most flattering angle --- it's lumpy and full of holes with only a hint of the X/Y/Z markings on the original shape).
![]()
We can also see that shrinkage of about 20 percent did in fact happen. The electrical resistance of the cube has also dropped to near zero.
Bound metal deposition printing at (lab)
Using filament and/or metallic resin and then casting if I get really desperate. Basically just trying to print metal
 Ahron Wayne
Ahron Wayne