New direction, lets make high grade nitiric acid. Watched a lot of YouTube videos about it. Unfortunately, there is only one method to make fuming nitric acid. The problem is it needs lots of lab equipment to perform the magic, and I want to make it simple (and cheap). So I thought of this design
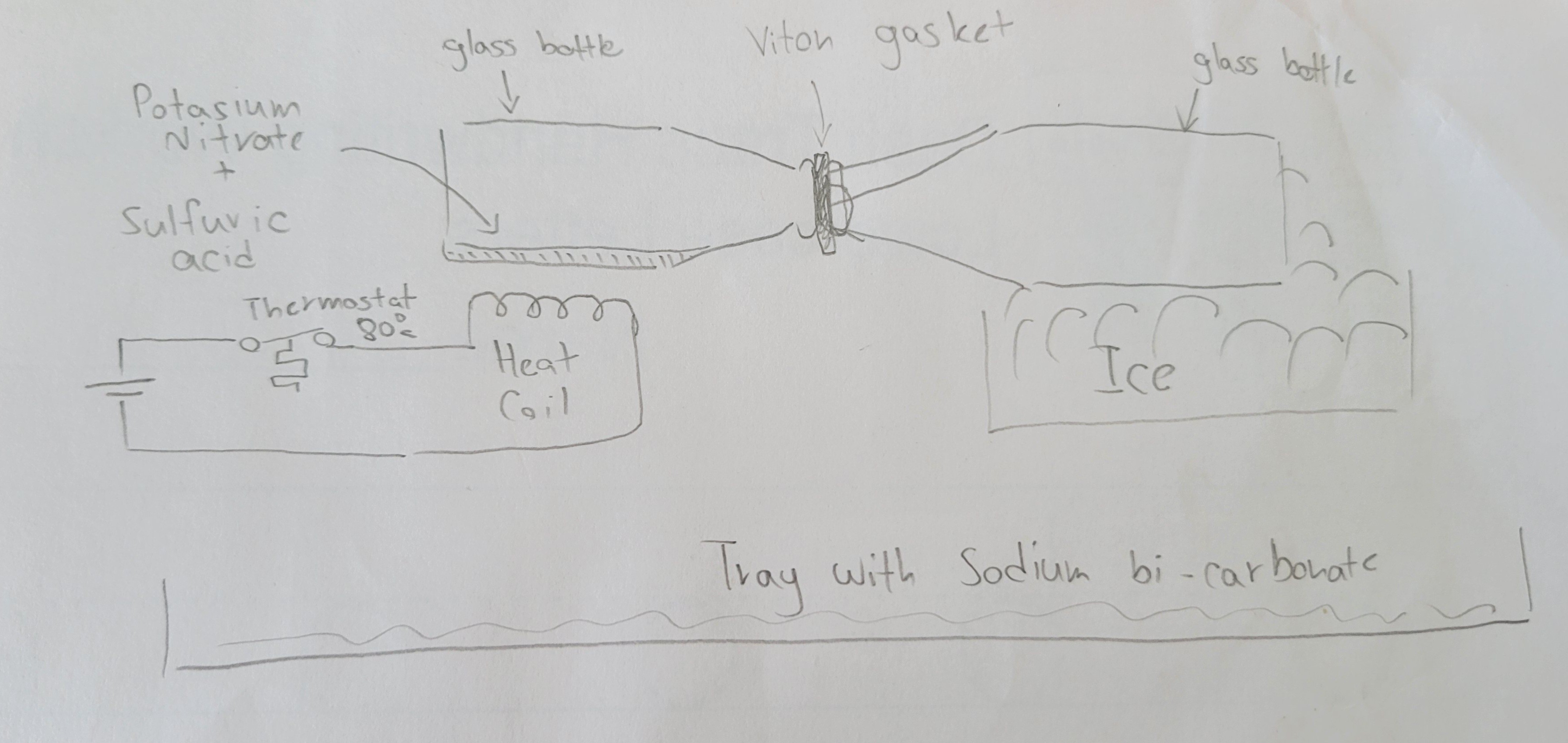
It's made of two beer bottles connected by Viton gasket. The idea is to have one bottle with Potassium nitrate+ Sulfuric acid. Other will be initially empty.
Then we apply heat to potassium+Sulfuric bottles while the empty one is chilled with ice. Potassium nitrate and Sulfuric acid makes Nitiric acid which is being heated up to 80°c (more can decompose Nitric acid) it diffuses to the other bottle as gas once it touches the cold bottle it should become liquid again. This way, after a while we will have one bottle with potassium bisulphate and other Nitric acid.
At the bottom we will place a tray with backing soda, in case of disaster. (we learned something from Chernobyl, didn't we).
Let's start with a dry test, instead of acids I will start with salty water. Let's check if there are leaks and how to open the bottles.
Made a connector to connect two bottles, it's more solid than I thought it would be.

One thing missing is a nice gasket between those bottles and we can run our first dry test.
It turned out that testing with water was a good idea. As I shortly found out, I had miscalculation on the width of the gasket. Another thing was the gasket is moving as I try to connect everything togather. I guess I will have to glue the gasket to the bottle.
At least the gasket fits.
Reprinting the forms with wider gap fixed most of the problem. I will try to heat water today and see how it goest.
Now I have glued the gasket to one of the bottles ( with salty water as it connected first).

It was much easier to connect everything with glued gasket.
Now let's run the dry-run experiment

With heat and ice on the other side. Rised temperature to 95°c so far so good nothing cracked nor exploded, yet.
Let's see the amount of water I get from this.
Ok, dry test was successful I got vapor and then water droplets on the empty bottle. One interesting insight is that most of the water condensed on the neck of the bottle.
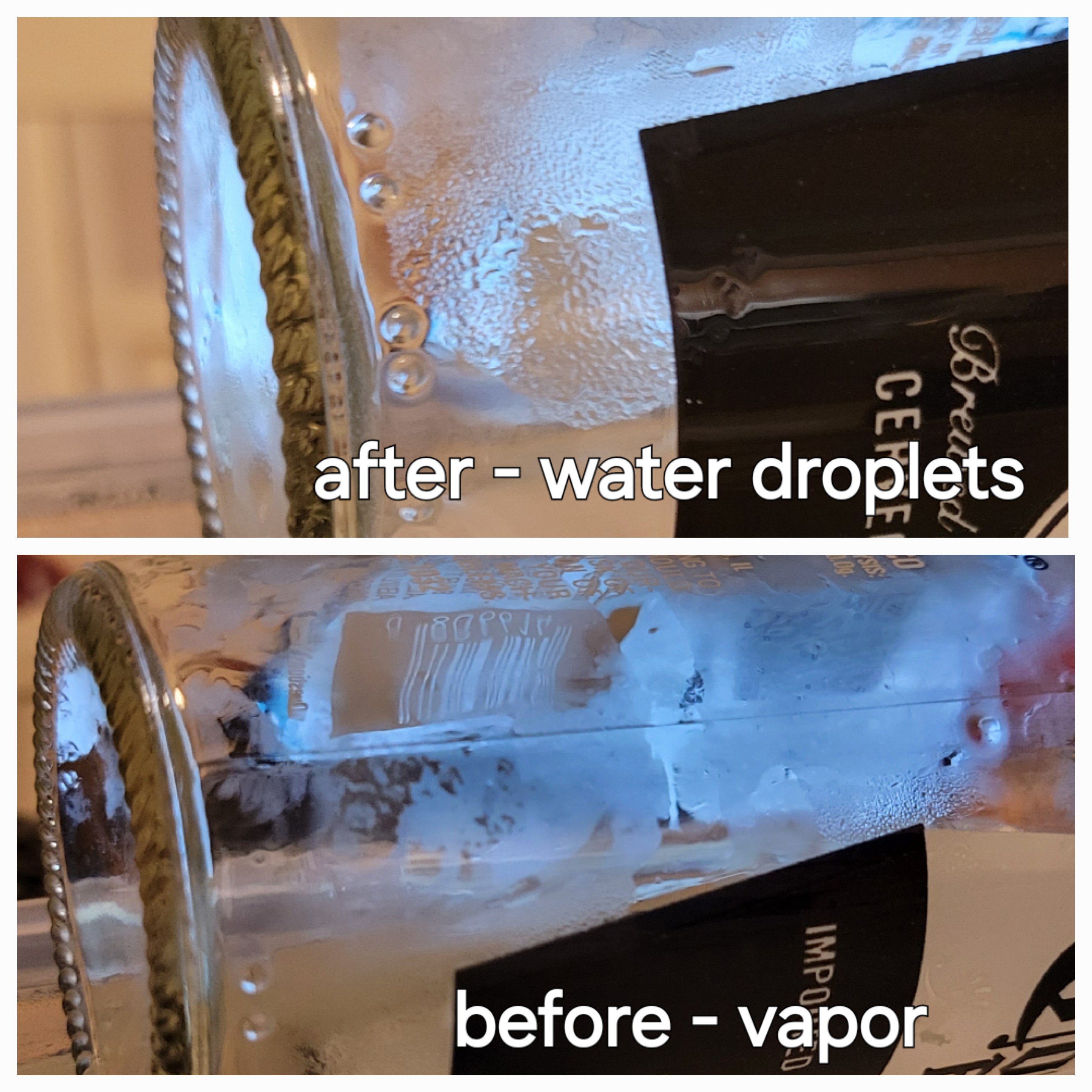
I don't worry about low quantity, few drops is all I need. I don't want to store Nitric acid. I need only few drops to crack open the package. All the other will be neutralized and discarded.
It's getting late, I will try to create fuming Nitric acid tomorrow.
Followed the same procedure like with water. Nothing happened, really NADA, not even moisture. I've started with 50°c went up to 65°c and finished with 83°c. Nothing.
After 30 minutes, I gave up.
Next time I will try to add much more Potassium nitrate and tilt the bottle to make vapor go down to ice cold bottle and condense.
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.