Safe IC decapping while preserving bond wires
Decapping using hardware store available materials and relatively safe procedure
Decapping using hardware store available materials and relatively safe procedure
To make the experience fit your profile, pick a username and tell us what interests you.
We found and based on your interests.
Use method #17 to get Nitric acid. Then use gentle method to get IC to the right temperature and use ultrasound bath to clean residue.
Since the beginning of the project I've never measured the temperature of IC but of the heating head, which is correlated but definitely not equal. Now I've made some simple Arduino based IR temperature sensor plus mosfet to control heating head temperature.
This way I can set 80°c and be sure that no acid is wasted and IC is not above it's maximal temperature.
Give me (more) fuel, give me (more) fire ...
Same as method #16 but with more powerful heating element.
First more heat
Now we can boil water in seconds
Also made samples with exposed bonding wires.
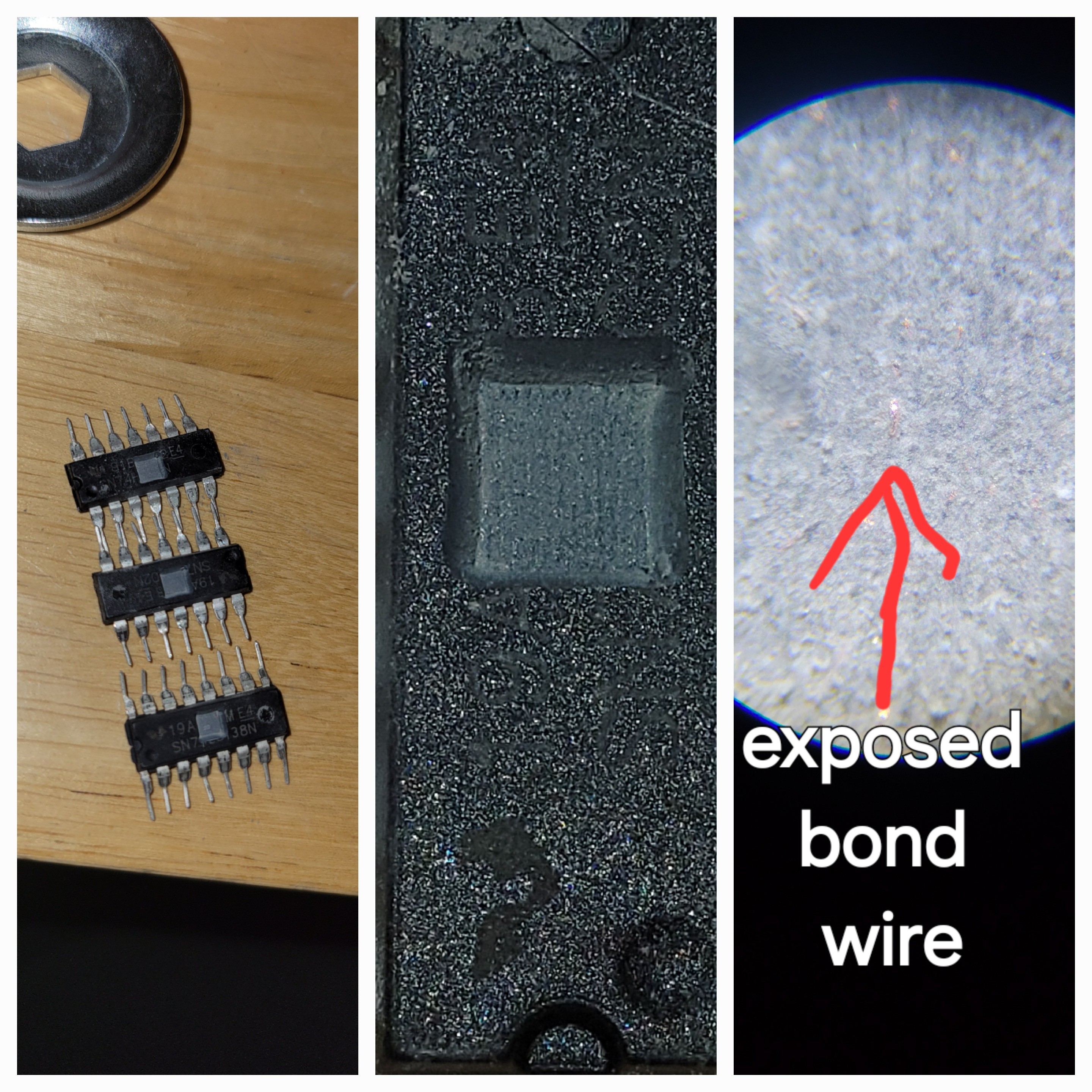
It will look like this
Some refactoring needed as the stove much wider plus I'll need to cover interior with aluminum foil to better distribute heat.(I don't want cardboard to catch fire)
It's always good idea to run dry test.
Compared to the old method the water started to condense almost immediately. The problem it was so hot the hot glue started to melt. I guess I need to run it on lower temperature
Things I did to reduce overheating:
* Reduced power from 5 to 2 on the stove.
* Used iced water
* Covered inside with aluminum foil to spread the heat evenly
Plus, I am using magnetic strips to open and close the box (And holding Nitric acid, we don't want it to fall on someone's head). Made of old 3D printer magnetic surface.
Run dry test, on water the water started to condense after few minutes and dropes were dropping in intervals of 3-5 seconds. The flask's arm was cold as I've hoped.
Now it's time to run real test...
I've started just like the dry test but nothing happened. Added heat insulation and drops started to appear. Got yellow liquid with terrible smell (yellow thing disappeared after few minutes). Once I stopped stove and let it cool down a bit, I've noticed there is rain outside. Some drops must go into Nitric acid :( but I had no choice but to continue.
The reaction was similar to one I saw earlier on method #16.
After some drops were maid I've cooled off everything and noticed I got too much acid. It took me forever to kill it with sodium bicarbonate.
But it was kind of fun
I still don't know if it failed because of drops of water or something else. I will re-run this experiment once never ending rain will stop.
Next time I will use much less Sulfuric acid. Plus I will try to put more potassium nitrate than Sulfuric acid. I have a suspicion that some of Sulfuric acid gets to output.
The result was successful (relatively), I've added first Potassium nitrate and then a little bit of Nitric acid. Everything was the same, except the color of acid was much more yellow
Although I put much less Sulfuric acid I got quite a lot of Nitric acid. (Almost same amount) after 30 minutes of work.
This time it reacted violently with terminals setting red fumes everywhere.
I was able to decap easily with only few drops of Nitric acid.
But again there is no bond wires, maybe because it used hot surface.
So next time I've tried to run with lower temperature. With lower temperature the epoxy turn to material felt like mud. I guess I've ruined the bond wires while trying to dig the 'mud'.
Another test I am running is using Nitric acid at room temperature. After 20 hours nothing happened.
Maybe the temperature was too high. I didn't see the green color that usually shows when copper bond wires dissolve. (When lead frame terminals dissolve there is red color).
I've put my acid on copper wire after first encounter it turned green when more acid added nothing happened, so it must be because a corrosion layer.
Also, checked with fluck, got 10 Ohm instead of 0.2 Ohm.
Looks like we are on the right track.
Then I've noticed something strange
I guess I knew it but ignored it. The hot plate is too hot for Nitric acid. It's just vaporized without doing anything useful, thus process is not efficient. Furthermore, this high temperature might brake bonding wire's connection.
I re-watched all the videos about making fuming Nitric acid. The first thing I thought ok, let's do it thr long (and right) way. Then, I realized that with such big flask and pipes and connectors I'll probably need full size fume chamber. Plus it will not be simple. After searching the web for different flasks I found this:
It gave me idea to make a different setup with same components.
Now we have two heat sources (it's ok since we get power from same 3D printer controller)
So we have to add another heat source, water pump and distillation flask. Except this we have already all the components.
Finally got the parts ☺️...
It should look something like that
Plus, I need to connect water pump to cool the arm of flusk.
Added cooling tube around the 'arm', plus, structure to hold it all together.

First dry test, I've poured some salty water into the flask(instead of Sulfuric acid). Some additional steps to be more efficient:
* Added some oil in the cup, for butter heat conduction.
* Wrapped in tin foil, otherwise condensed water get cooled off from flusk walls and goes down.
* It took me 30-40 minutes to get first drops.
* Used maximal temperature I can squeeze from this tiny heating element. (It's limited to 260°c by software)
One of the problems I've observed was creation of a bubble on the arm/output. Instead of dripping the water out it just accumulated it. It's strange given the fact that it diameter is about 3-4mm. Maybe I should lower it down to increase angle.
Another problem was aiming at this tiny 3x3mm area milled inside the package.
In this experiment the top opening was not closed, it got dump after while, maybe I should seal it Teflon.
The good things were that tube and it's glue that holds it (I've used hot glue, probably not the best idea but it's good for prototyping) is not getting hot AND nothing exploded. My primary concern was that it will blow up when there is such big temperature difference.
No pictures except this short video
From dry run I've learned that it's will be hard to drop the acid right in it's place so I've added little spout from Viton rubber. (The tape should not touch the acid, it's there to maintain spoiut's V-shape)
Now left the enclosure. For now it has lots of parts some should be removable for cleaning.
Still haven't figured out how to make easy to remove
Ended up with simple design
Most important is getting rid of sand only usong oil for better heat transfer. And socks to keep heat in
This thing with sock on it reminds me of RHCP ;) nevermind...
In the end I was able to produce something that looks like nitirc fume and few drops of acid. I still have to find way to make it faster.
Place one drop on IC (no heating) was able to see some etching of epoxy.
Trying without second heater and smaller design with better angle. I hope this will yield more acid.
Better angle and better heat insulation resulted in more acid produced something of magnitude of few drops. Although it's much less than I expected it's good enough for me at this point as I don't need much of Nitric acid.
It's probably Nitric acid since Sulfuric acid's boiling temperature is 340°c and Nitiric 83°c. Plus the drops are of clear white color. (It's not water as it reacted violently with sodium bicarbonate)
I put a drop on an IC with cavity and got nice bubbles. It was not as powerful as I expected.
It took me few iterations to do something and I was out of acid before I got to bonding wires.
So I don't know if it works or not... 😕 I guess I need more powerful heating element.
Just like #14 but with much more potassium nitrate.
I've added much more Potassium nitrate and even with no heat I got some droplets. Now applied heat for 45mins, let's open it up and see how much I got.
There are some drops but I was unable to get them.
The potassium nitrate was wet I guess it has a lot of Nitric acid to produce. Probably I should apply much more heat. But if I apply more heat ot will break the connector that I 3D printed.
I guess I should use the glass and breakers everybody uses.
New direction, lets make high grade nitiric acid. Watched a lot of YouTube videos about it. Unfortunately, there is only one method to make fuming nitric acid. The problem is it needs lots of lab equipment to perform the magic, and I want to make it simple (and cheap). So I thought of this design
It's made of two beer bottles connected by Viton gasket. The idea is to have one bottle with Potassium nitrate+ Sulfuric acid. Other will be initially empty.
Then we apply heat to potassium+Sulfuric bottles while the empty one is chilled with ice. Potassium nitrate and Sulfuric acid makes Nitiric acid which is being heated up to 80°c (more can decompose Nitric acid) it diffuses to the other bottle as gas once it touches the cold bottle it should become liquid again. This way, after a while we will have one bottle with potassium bisulphate and other Nitric acid.
At the bottom we will place a tray with backing soda, in case of disaster. (we learned something from Chernobyl, didn't we).
Let's start with a dry test, instead of acids I will start with salty water. Let's check if there are leaks and how to open the bottles.
Made a connector to connect two bottles, it's more solid than I thought it would be.
One thing missing is a nice gasket between those bottles and we can run our first dry test.
It turned out that testing with water was a good idea. As I shortly found out, I had miscalculation on the width of the gasket. Another thing was the gasket is moving as I try to connect everything togather. I guess I will have to glue the gasket to the bottle.
At least the gasket fits.
Reprinting the forms with wider gap fixed most of the problem. I will try to heat water today and see how it goest.
Now I have glued the gasket to one of the bottles ( with salty water as it connected first).
It was much easier to connect everything with glued gasket.
Now let's run the dry-run experiment
With heat and ice on the other side. Rised temperature to 95°c so far so good nothing cracked nor exploded, yet.
Let's see the amount of water I get from this.
Ok, dry test was successful I got vapor and then water droplets on the empty bottle. One interesting insight is that most of the water condensed on the neck of the bottle.
I don't worry about low quantity, few drops is all I need. I don't want to store Nitric acid. I need only few drops to crack open the package. All the other will be neutralized and discarded.
It's getting late, I will try to create fuming Nitric acid tomorrow.
Followed the same procedure like with water. Nothing happened, really NADA, not even moisture. I've started with 50°c went up to 65°c and finished with 83°c. Nothing.
After 30 minutes, I gave up.
Next time I will try to add much more Potassium nitrate and tilt the bottle to make vapor go down to ice cold bottle and condense.
I am trying to remove the white layer.
I thought I can use Citrix acid, to remove. I hopped it will passivate the bond wires.
It looked promising but then I found out that Citrix qcid dissolve copper. It's not passivation layer it the shell of dead bonding wire.
I got all the pieces of the puzzle figured out.
Basically the decapping is done in three steps
1. Get rid of package's upper level until you see the top part of bonding wires. This can be done with cheap low power laser. I have 5W optical laser for 50$. I use only about 1% of it's power.
After few iterations of scorching by laser and scrapping the glass material. You get the bonding wires.
2. Coat bonding wires with some material that will oxidize copper. Thus, making protection layer against chemicals like diluted Nitric acid.
I've used NaOH (just because I am familiar with it) there are two coils one that was connected to anode other to cathode. The copper coil that was connected to anod was dissolved even without heat. The cathode's coil stayed intact.
Another test I've run was making oxidation layer using plain water.
The coil that was connected to anod (+) was coated with oxidation layer and was not destroyed by Nitric acid. The other one got rust and braked easily once I tried to remove it.
3. Use whatever nitiric acid you can get
That's what we already did in last eleven attempts.
P.S.
Although the idea of electric oxidation/coating is nice practically it's a problem to do something in such a small vicinity.
I've tried to use higher temperatures of 260°c for short time of 2-3 minutes. It was late at night and it didn't occur to me to check Fuming Nitric acid boiling temperature which is only 83°c. (Concentrated boiling point is 120°c) Basically I've vaporized all Nitric acid before it could do anything.
Like method #9 but instead of making diy Nitric acid in epoxy pocket, heated materials in a beaker and apply it on the IC. Still there is crystal like residue formed.
I don't have a clue how to continue this project 😭
--------------------------------------------
OK, after watching some videos and asking forums I got my answer, my problem is using NOT concentrated nitric acid (duh!)
This video explains why it should work with highly concentrated nitric acid (AKA fuming nitric acid) is ok with copper bond wires. (TL'DR go to 4:05)
The same as method #8 but not washing with water but aceton.
I've probably broke the bonding wires with cnc machine but it's doesn't matter, what is meters is that bonding wires got spiky meaning they were etched...
Create an account to leave a comment. Already have an account? Log In.
It looks like your experimentation with Citrix acid did not go as planned, especially with its effect on copper rather than just removing the white layer. Your step-by-step approach, especially the laser and oxidation processes, shows detailed thought and troubleshooting. However, dealing with such small components seems to present challenges, particularly when it comes to precise application of electric oxidation. Your persistence is commendable—keep experimenting, but maybe consider revisiting the chemical compatibility to protect the bonding wires better!
Thank you for kind words, if you have some ideas about passivation I will be glad to learn/test.
Become a member to follow this project and never miss any updates
Informative