I re-watched all the videos about making fuming Nitric acid. The first thing I thought ok, let's do it thr long (and right) way. Then, I realized that with such big flask and pipes and connectors I'll probably need full size fume chamber. Plus it will not be simple. After searching the web for different flasks I found this:

It gave me idea to make a different setup with same components.
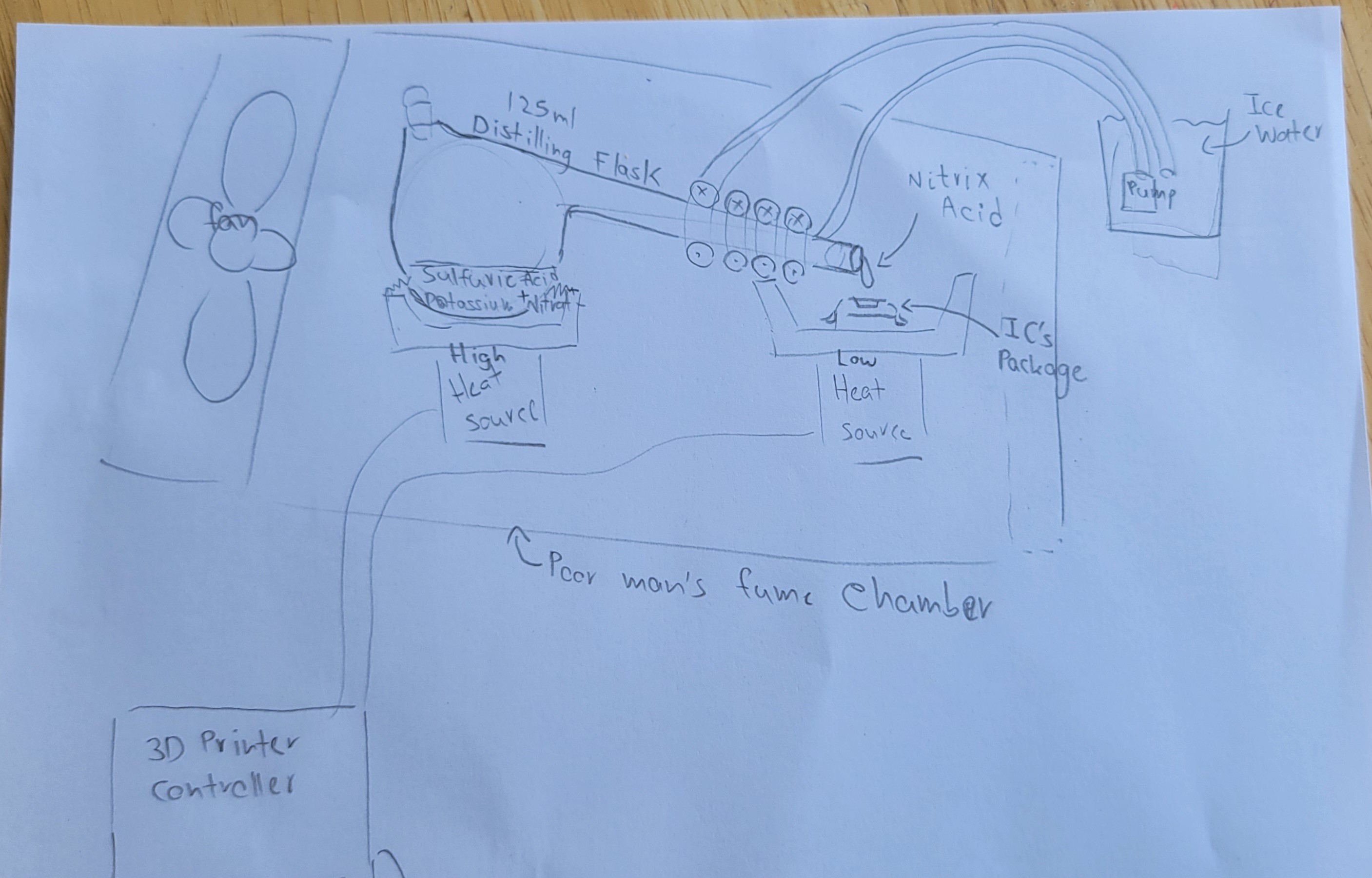
Now we have two heat sources (it's ok since we get power from same 3D printer controller)
So we have to add another heat source, water pump and distillation flask. Except this we have already all the components.
Finally got the parts ☺️...
It should look something like that

Plus, I need to connect water pump to cool the arm of flusk.
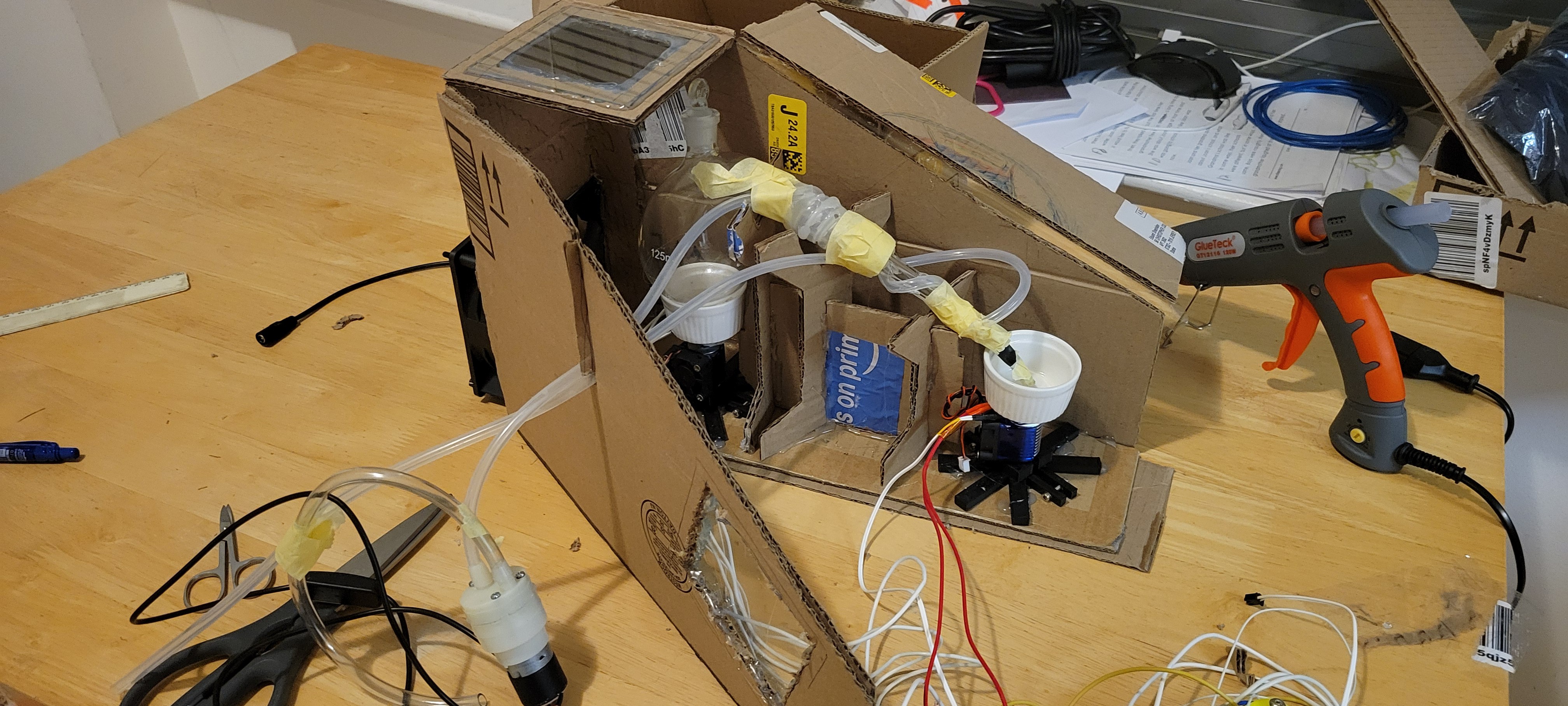
Added cooling tube around the 'arm', plus, structure to hold it all together.

First dry test, I've poured some salty water into the flask(instead of Sulfuric acid). Some additional steps to be more efficient:
* Added some oil in the cup, for butter heat conduction.
* Wrapped in tin foil, otherwise condensed water get cooled off from flusk walls and goes down.
* It took me 30-40 minutes to get first drops.
* Used maximal temperature I can squeeze from this tiny heating element. (It's limited to 260°c by software)
One of the problems I've observed was creation of a bubble on the arm/output. Instead of dripping the water out it just accumulated it. It's strange given the fact that it diameter is about 3-4mm. Maybe I should lower it down to increase angle.
Another problem was aiming at this tiny 3x3mm area milled inside the package.
In this experiment the top opening was not closed, it got dump after while, maybe I should seal it Teflon.
The good things were that tube and it's glue that holds it (I've used hot glue, probably not the best idea but it's good for prototyping) is not getting hot AND nothing exploded. My primary concern was that it will blow up when there is such big temperature difference.
No pictures except this short video
From dry run I've learned that it's will be hard to drop the acid right in it's place so I've added little spout from Viton rubber. (The tape should not touch the acid, it's there to maintain spoiut's V-shape)

Now left the enclosure. For now it has lots of parts some should be removable for cleaning.

Still haven't figured out how to make easy to remove
Ended up with simple design
Most important is getting rid of sand only usong oil for better heat transfer. And socks to keep heat in

This thing with sock on it reminds me of RHCP ;) nevermind...
In the end I was able to produce something that looks like nitirc fume and few drops of acid. I still have to find way to make it faster.
Place one drop on IC (no heating) was able to see some etching of epoxy.
Trying without second heater and smaller design with better angle. I hope this will yield more acid.

Better angle and better heat insulation resulted in more acid produced something of magnitude of few drops. Although it's much less than I expected it's good enough for me at this point as I don't need much of Nitric acid.
It's probably Nitric acid since Sulfuric acid's boiling temperature is 340°c and Nitiric 83°c. Plus the drops are of clear white color. (It's not water as it reacted violently with sodium bicarbonate)
I put a drop on an IC with cavity and got nice bubbles. It was not as powerful as I expected.
It took me few iterations to do something and I was out of acid before I got to bonding wires.
So I don't know if it works or not... 😕 I guess I need more powerful heating element.
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.