Executive Summary
Problem: In the United States, there are 143,000 individuals who have lost the use of their dominate hand due to a stroke. Of these, approximately 93,000 will have not successfully recovered the use of their hand after 6 months of treatment. Experts suggest that this lack of recovery is due to a lack of data. This lack of data contributes to poor success rates because (1) reimbursements for care at these centers is dependent upon quantifiable results, (2) the optimal intensity of training cannot be determined without specific data to quantify the patient’s progress, and (3) patients lose motivation without concrete evidence that they are making progress.
Solution: In response, Purdue MIND created the ExoMIND Glove. This glove will meet the identified needs by (1) facilitating the creation of quantitative reports of therapeutic progress to allow for complete reimbursements, (2) allowing physical therapists the ability to identify and address specific deficiencies in the patient’s therapy, and (3) providing robotic rehabilitation solutions to patients struggling to regain use of their hands.
Differentiation: Due to the narrow focus of these devices on patient recovery, other stakeholders, specifically the acute care center’s needs, have not been adequately addressed. The ExoMIND Glove presents the first attempt in this market to provide acute care centers with holistic analytical reports to track the progression of the patient’s recovery during acute rehabilitation care.
Prototype of final design
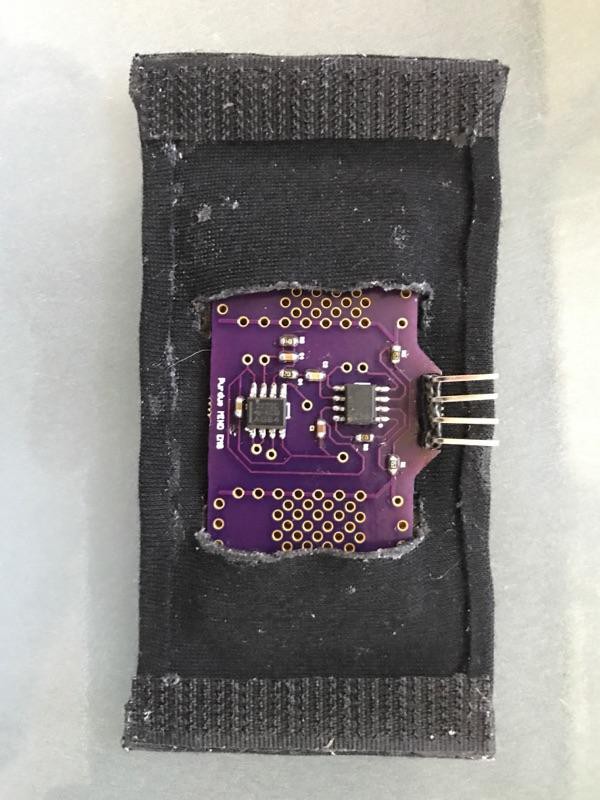
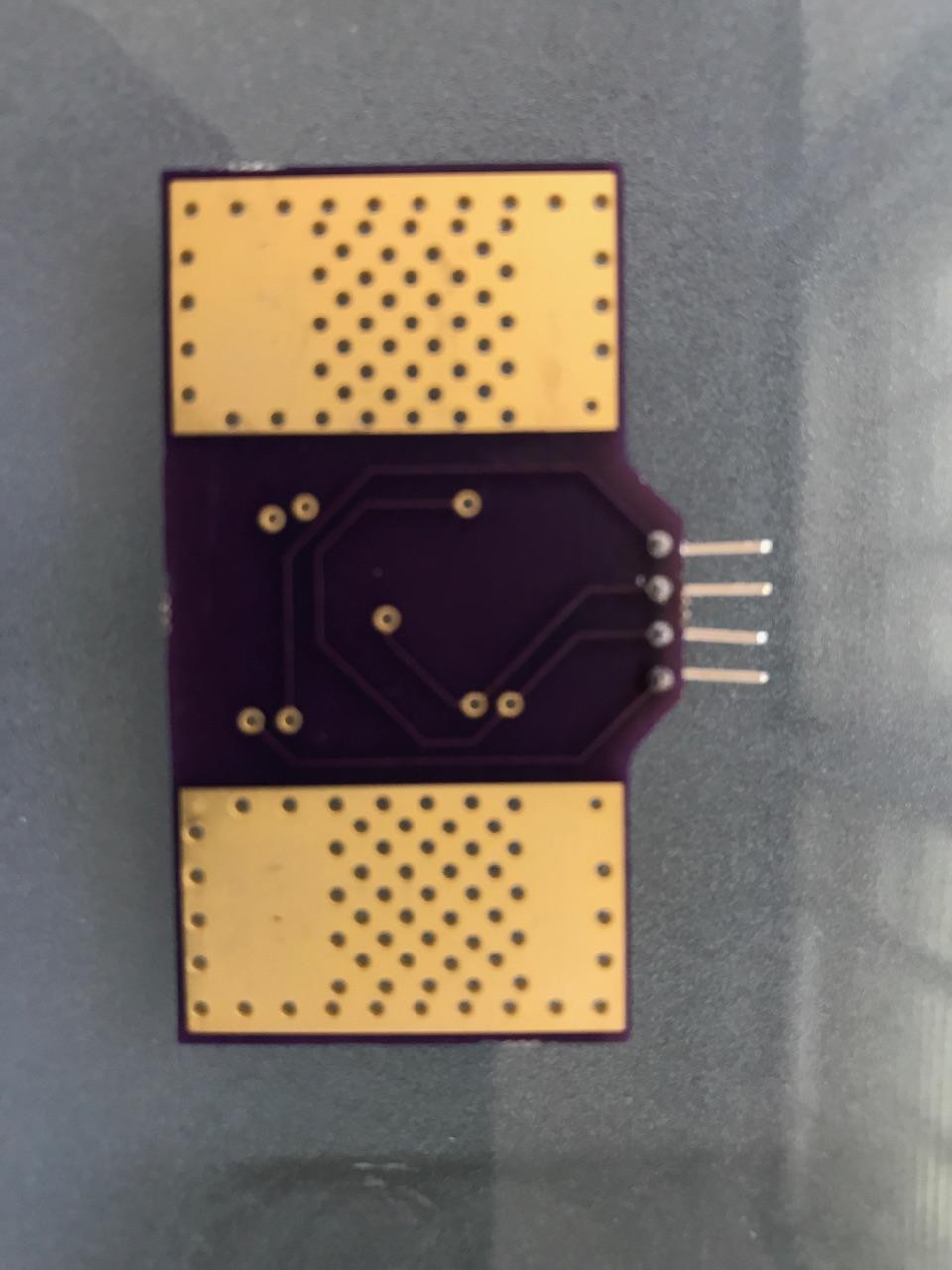
Our solution utilizes seven velcro ring accelerometers and one fabric bipolar electrode EMG sensor unified within a single adjustable glove that slides over the user’s forearm and hand to allow ease of use. The glove is equipped with one EMG sensor sewn into the device with conductive silver fabric to comfortably measure electrical activity produced by skeletal forearm muscles. This EMG sensor is wired to a central housing unit (i.e. a breadboard) that is mounted on the top of the device. Five ring accelerometers housed in 3D printed units are mounted on each of the user’s finger tips to measure relative finger position. The remaining two accelerometers measure wrist motion by analyzing the position of one accelerometer on the back of the hand relative to an accelerometer in the central housing unit on the user's forearm. All accelerometers are wired to the central housing unit without impeding the user’s range of motion. Contained within the central housing unit is an Arduino Pro Mini which is paired with a software that implements an interactive program to walk the user through proper usage of the ExoMIND Glove.
Documentation of final design
Accelerometers
The circuitry of the accelerometers were designed and developed on custom printed circuit boards (PCBs) via Eagle software and sent to OSHPark to print for use. Due to these criteria, the MPU6050 was used for all accelerometers. The accelerometers detect changes in the angle of each fingertip with respect to a baseline position of a closed fist by using a reference accelerometer fastened to the backside of the patient’s hand. These values must be used to obtain a measure of the range of motion of each individual finger such that a physician or physical therapist may quantify and personalize the therapy over time by identifying which fingers or types of exercises need more direct attention. The user needs a simple and interactive program to walk the user through proper usage of the device to ensure accurate readings and clearly displayed results.
Electromyograms
The analog front end (AFE) and bipolar electrodes of the EMGs were designed and developed on custom printed circuit boards (PCBs) via Altium software. The AFE and electrode design had to be small enough to be comfortably worn within a wrist brace encapsulating the forearm, and to filter frequencies outside the 70 Hz - 1590 Hz range while amplifying the differential signal by 1000x to meet design specifications. The dry...
Read more » skuhns
skuhns