-
More on artificial lighting
04/13/2018 at 21:01 • 0 commentsIn an earlier log entry I made about artificial lighting, I wasn’t sure how efficient the LEDs I was using were, and so I didn’t really know how much light the plant that was under them was receiving.
A lot of LED datasheets don’t contain data directly about the efficiency of the LED – i.e. how much light energy the LED outputs per unit of electrical energy.
I found this useful post about how to calculate/estimate the efficiency of an LED from the datasheet parameters: https://electronics.stackexchange.com/questions/325949/how-can-i-estimate-the-optical-power-that-a-single-color-led-generates
For example, the red LEDs I had been using for previous experiments is a Cree C503B-RCS 624nm Red 5mm dome LED.
The forward voltage is 2.1V, so with a forward current of 20mA the LED consumes 42mW
The luminous intensity is 6.6cd. The 50% power angle of the LED is 30 degrees, so lets say that the intensity is on average 4.95cd within the 30 degree angle, and ignore anything outside of this.
The solid angle is 2*pi*(1 – cos 15 degrees) = 0.214 steradians, so the luminous flux of the LED is 4.95*0.214 = 1.06 lumens.
The luminousity function at 624nm is 0.333 (obtained from http://www.ies.org/definitions/table-134-definitions/table-134-definitive-values-of-the-special-luminous-efficiency-function-for-photopic-vision-v/ ). The radiant flux is therefore 1.06 / (683*0.333) = 4.66mW, so the efficiency of the LED is 4.66/42 = 11 percent.
Realising that this LED wasn’t very efficient, and also can’t tolerate sustained currents of more than about 30mA, I ordered some more efficient LEDs that can take a higher current: the OSRAM OSLON Signal 120 LJ CKBP JZKZ 25-1-35. The datasheet gives the luminous intensity, but it also gives the luminous flux, and in one place it gives a figure for efficiency, so I can use these to check my estimates. The calculations below all assume a forward current of 350mA because that’s what the datasheet parameters are based on, but when I use this LED I’ll actually be using less current than this.
The forward voltage is 2.15V, so with a forward current of 350mA the LED consumes 753mW
The luminous intensity is typically 21.8cd at 350mA. The 50% power angle of the LED is 125 degrees, so lets say that the intensity is on average 16.4cd within the 125 degree angle
The solid angle is 2*pi*(1-cos 62.5 degrees) = 3.38 steradians, so the luminous flux of the LED is 16.4*3.38 = 55.4 lumens.
The data sheet doesn’t give the typical value for the luminous flux, but it gives a minimum and maximum value, and the average of these is 66 lumens, so my estimate is low (perhaps because I haven’t accounted for anything outside the 125 degree angle) but not too far off.
The luminosity function at 625nm is 0.321, the radiant flux is therefore 66 / (683*0.321) = 301mW, and the efficiency of the LED is 301/753 = 40 percent.
In one place in the datasheet (under text for the “Electrical thermal resistance junction / solder point”) an efficiency figure of 38% is mentioned, so my calculation is not far from that.
-
Fifth attempt
04/08/2018 at 21:13 • 4 commentsIn the fourth attempt one of the seedlings developed a true leaf, but all seedlings died 5 weeks after planting. I believe that the cause of death was excessive CO2 concentration in the bottle.
In this experiment (started on 5th April) I'm taking an additional measure to reduce the rate at which the soil releases CO2. I have the same amount of water in the bottle (12ml), but I'm replacing some of the soil with green sand. The diagram below shows the setup for this experiment. The photo beneath this shows the layer of green sand above the soil. I’ve used green sand rather than ordinary sand because I believe it will hold more water than ordinary sand.![]()
![]()
I made the green sand by mixing some general purpose sand with powdered bentonite clay (from clumping cat litter). Note that no additional water was added to the green sand - it was a dry mix of 20g sand with 2g bentonite clay.
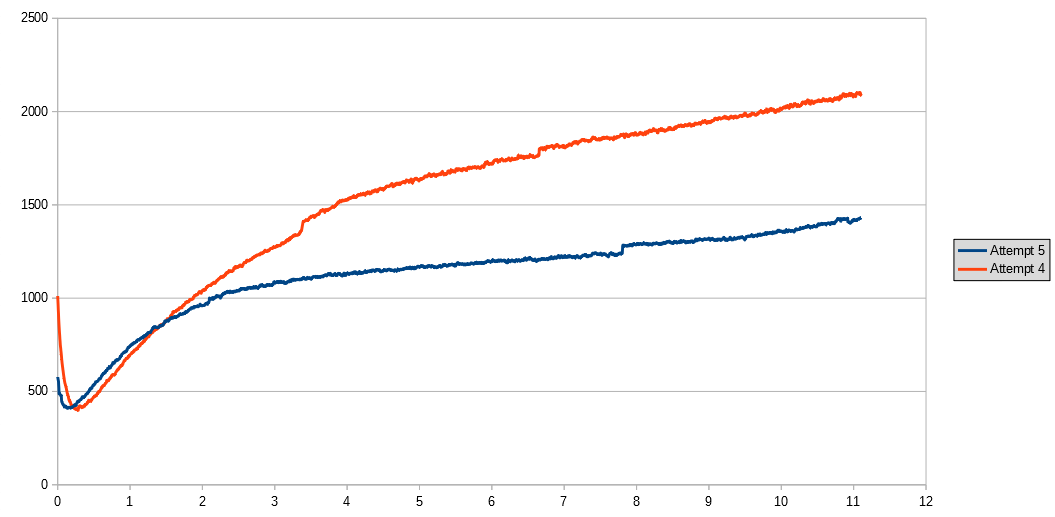
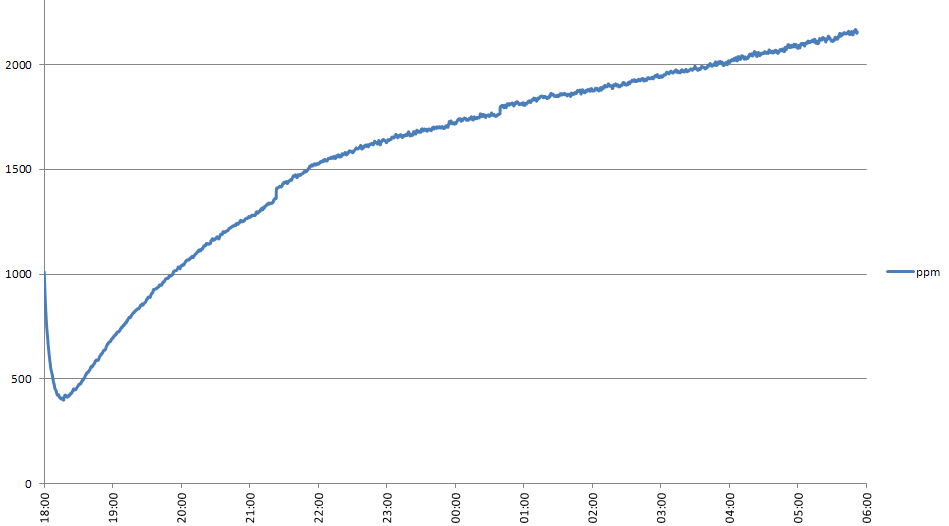
The idea is that some of the initial water in the bottle will be absorbed by the green sand, which won't contribute to CO2 production. The graph below shows the CO2 concentation for the first 11 hours of this experiment, compared with the fourth attempt. The graph does show a slower rate of CO2 release in this experiment. I've also measured the initial CO2 level for a longer period of time than in the previous experiment - I'll post details of this in a few days.
![]()
It remains to be seen whether the sand will affect seed germination. The seeds (3 this time rather than 5, because I believe that having 3 still gives a good chance that at least one will germinate and begin to grow) were placed into dimples in the sand and covered over with a small amount of damp garden soil to introduce microbes (I haven't yet experimented to see whether it is really necessary to cover over with damp soil - perhaps some microbes survive in the soil that I dry out on the radiator prior to putting it into the bottle?). In previous experiments the seedlings took about 8 days to appear, so I expect to see them on about 13th April.
UPDATE 13th April 2018
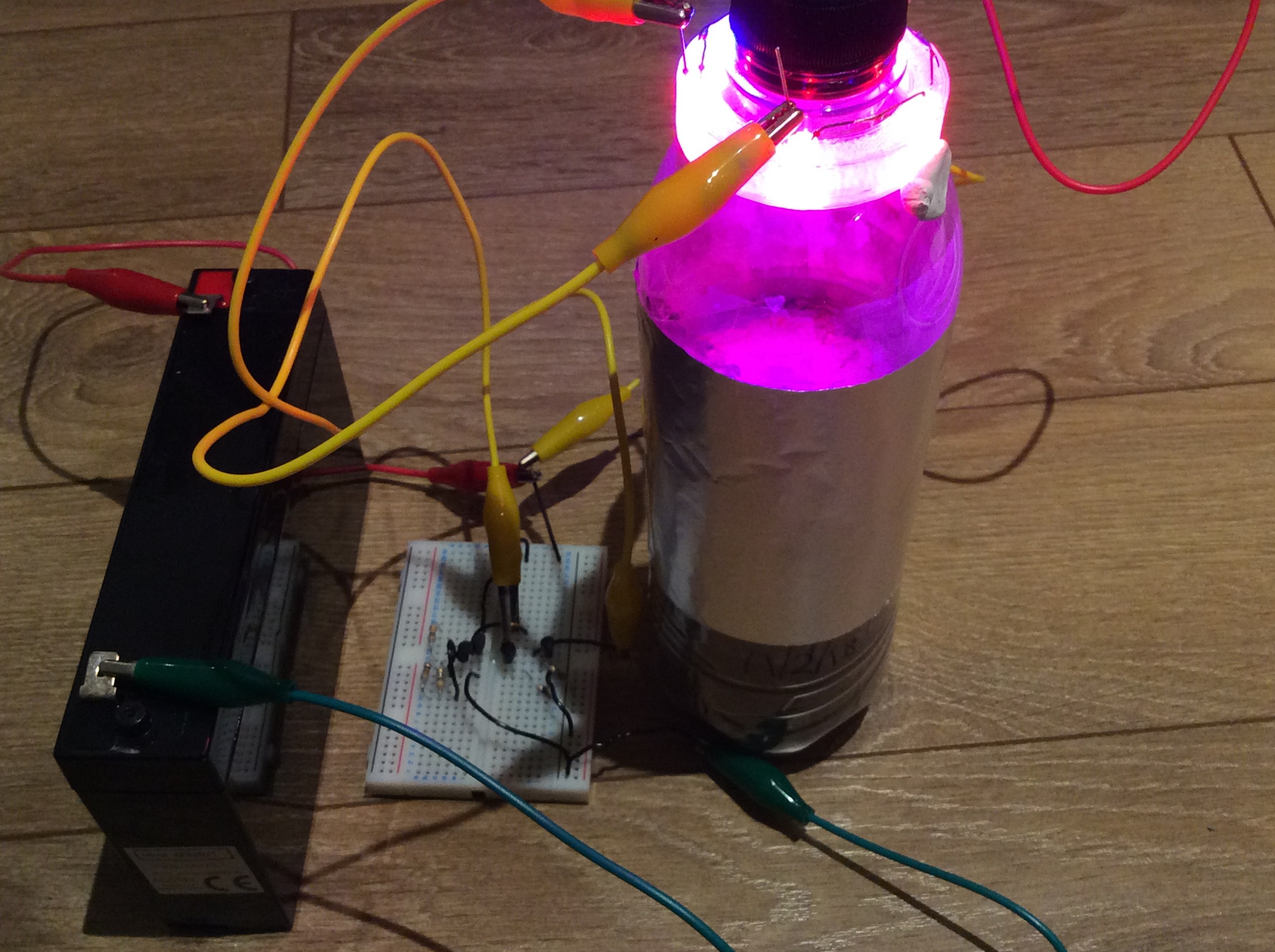
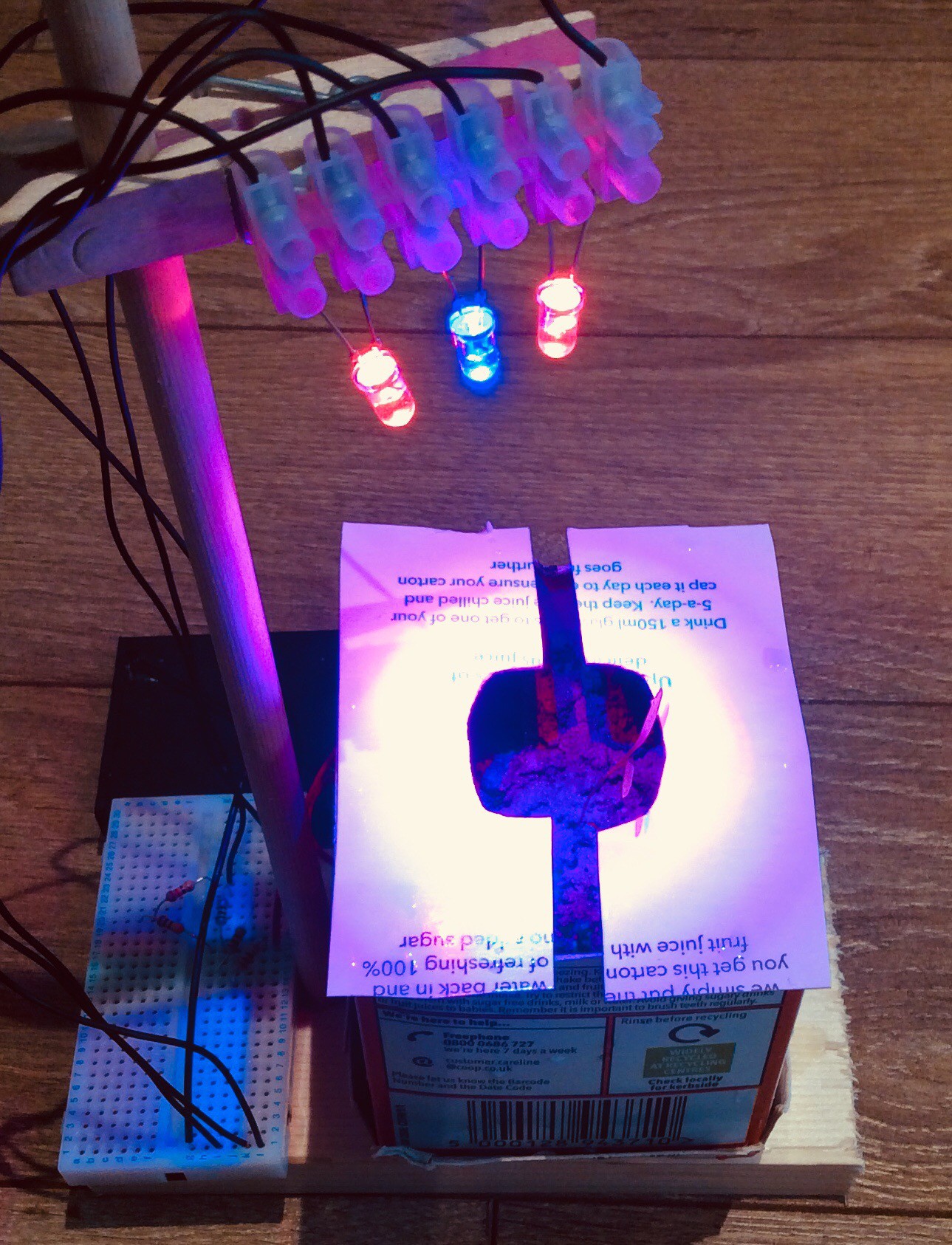
The first green shoot was visible this morning. I’ve added LED lighting to this bottle, using brighter and more efficient LEDs than I’ve used previously (see log entry ‘More on artificial lighting’). I’ve used 3 red LEDs and 1 blue LED. I believe that the illuminated area is receiving about 100 W/m2. The LEDs are mounted inside the bottle. I had wondered whether using more powerful LEDs would cause the bottle to heat up too much. I measured the temperature increase at the surface of the soil and it was only about 1 degree C above the temperature in the room. The LEDs are controlled by a timer circuit which switches them on for 18 hours then off for 6 hours. Reflective mylar is wrapped around the bottle to help prevent light from escaping.
![]()
![]()
UPDATE 27th April 2018:
The first true leaf has appeared on one of the seedlings, first noticed on 25th April (20 days after planting). I’ve also noticed that the stems of the seedlings are redder than in previous experiments.
![]()
Note that the intention behind the three holes cut into the reflective mylar foil sheet was that one seedling would grow in each hole. One of the seeds didn’t germinate, and the seed in the middle hole ended up sprouting from the neighbouring hole, so although the two seedlings in the photo appear to be growing from the same place, but one has its root over towards the middle hole.
UPDATE 8th May 2018:
The seedlings are both still standing and a true leaf has appeared on the smaller of the two. The red colouration of the leaves has steadily increased, so the seedlings are now very dark red. I don’t know whether this is an effect of the light, heat from the LED, or the green sand. Apart from the red colour, they are at a similar stage of development to the carrot seedlings I planted outside over the weekend, which were grown in the conventional way.
![]()
UPDATE 14th May 2018
The seedlings are both still alive today, 39 days after planting, so have lived for longer than in any of the previous experiments, and are both more fully developed than in previous experiments. No second leaf has yet appeared on the larger seedling, and the seedlings are both still red. I have read about possible causes of red leaves in plants, and I believe that it could be because there is not enough water in the soil for the roots to grow very far. Below is an image of two of the roots. One root can be seen near the top left of the soil, within the dampest part of the soil. The other is growing diagonally downwards from the middle right of the soil, down into the dryer part of the soil.
![]()
I have deliberately restricted moisture in the soil to prevent CO2 overproduction, but now it seems that it may be a limiting factor. What can be done about this in future experiments? One possibility is to put water in the stoney layer at the bottom of the bottle - there it won’t contribute to initial CO2 production, but it will slowly evaporate and condense elsewhere in the bottle, and ultimately make the soil damper.
UPDATE 31st May 2018:
It’s now 56 days since the seeds were planted. The seedlings are still alive, but there has been no noticeable growth for about 2 or 3 weeks. The only obvious change is that the roots are more obvious around the edge of the container - I believe that the roots have continued to develop. My guess is that the seedlings don’t have enough water. Because the amount of water produced by soil microbes is proportional to the amount of CO2, the level of CO2 in this bottle probably won’t ever get too high now: any extra water/CO2 produced by the soil will be used immediately by the seedlings. Rather than continue this experiment as it is, I’m going to measure the CO2 concentration in the bottle, then add some additional water and see whether that causes the seedling to continue to grow, and if so then whether it will also revert to a green colour (at the moment there is still some green visible in the true leaf of the larger of the two seedlings).
![]()
![]()
UPDATE 5th June 2018:
I measured the CO2 concentration today and it was greater than 10000ppm. I also added 24ml of water to see whether this will enable the seedling to resume it’s growth. Some CO2 will have escaped from the bottle when I removed the cap to make this measurement and add the additional water - I attempted to minimise the length of time that the cap was off.
In reply to one of the comments below, here are photos of carrots growing in the open air in similar sized containers to a 600ml bottle. The first two pictures show a carrot that has been growing on a window ledge in a jar. In fact it’s the same one that I started growing in the log entry entitled “Artifical Lighting”: I replanted it in a jar and left it on the window sill after that. I was surprised at how many small roots there are in the jar, I didn’t realise that carrots have so many smaller roots in addition to the main enlarged orange tap root.
![]()
![]()
The next photo shows a carrot that’s been growing outside in a small cardboard container:
![]()
The root has thickened, even though there’s very little space. A couple of the leaves of this one turned reddish.
UPDATE 11th June 2018 (66 days after planting):
The effect of adding the additional 24ml of water was apparent after a couple of days. Now, 6 days later, it is very obvious. The first true leaf on the smaller of the two seedlings has grown substantially, and is green, except at the tips of the leaves. Both seedlings have developed a second true leaf (the second leaf on the smaller seedling is still small) and the new leaves are green:
![]()
FINAL UPDATE
The photo below shows what the seedling looked like on the 23rd July. It didn’t die, but didn’t develop a very large root either. This years in-season carrots have just run out, so I’m planning to do some more experiments this winter.
![]()
-
Measuring CO2 loss from a bottle when the lid is removed
02/20/2018 at 07:57 • 0 commentsSometimes I want to be able remove the lid from a sealed bottle to attach a CO2 sensor, then put the lid back on afterwards. How much CO2 is lost from the bottle when I do this?
I performed the experiment as follows:
- CO2 level in the room at the start of the experiment was measured as 400ppm
- Temperature in the room at the start of the experiment was measured as 16.1C
- Breathed into 600ml bottle to raise CO2 level.
- Left sensor attached to bottle for 1 hour (to make sure the reading had stabilised)
- Removed sensor for approximately 10 seconds, then reattached. Removal and reattachment involve screwing and unscrewing. 10 seconds was the duration of the period when the opening of the bottle was unobscured by the sensor and open to the air in the room.
- Left the sensor attached until the reading was stable again.
- Temperature in the room at the end of the experiment was 16.3C
- CO2 level in the room at the end of the experiment was still 400ppm.
The CO2 level measured at the end of step 4, before exposing the bottle to open air, was 9600ppm. At the end of step 6 the reading was 9100ppm.
I believe that 10 seconds is longer than the total time that the bottle opening is exposed to the air when I normally attach then later remove the CO2 sensor. In most of the experiments I’m doing, the volume of air in the bottle is probably between 300ml and 400ml, the CO2 level in the bottle is generally less than 10000ppm, and the CO2 level in the room is between 400ppm and 1300ppm. So I think I lose 5-10% of the CO2 in the bottle when I attach and remove the sensor.
-
Fourth attempt
02/15/2018 at 08:36 • 0 commentsThis experiment (started on 11th Feb 2018) is like the experiment described in the log entry ‘Third Attempt’ dated 4th Jan 2018. There are a few differences. I haven’t used any moss this time. The moss introduced unintended seeds into the third attempt. Also, after planting the carrot seeds and watering with 12ml water, I put the bottle outside for 1 hour to vent off the initial carbon dioxide release that I believe happens when water is put onto dry soil. After 1 hour I brought the bottle back indoors and quickly connected the CO2 sensor. The graph below shows the result:
![]()
The CO2 sensor reading drops initially because the sensor had previously been measuring the indoor CO2 level, which is usually slightly above 1000 ppm when I’m in the room. The minimum reading was 420ppm, which is consistent with what I expect the outdoor CO2 level to be, given that the sensor was attached to the bottle within about twenty seconds of bringing it indoors. The CO2 level rose rapidly at first, then the increase levelled off to a rate of about 500ppm every six hours. I disconnected to CO2 sensor in the morning, and quickly put the bottle lid back on so that not too much CO2 diffused out.
By the time the seedlings start to appear and start photosynthesising, it seems that the CO2 level will be between 10000 and 20000 ppm - this may be too high.
I have LEDs attached to the bottle, as shown in the photo below, so that I can provide enough light for the seedlings once they start to grow, by which time the control circuit will be soldered onto stripboard. The bottle has some reflective aluminium foil wrapped around part of it to reflect some of the light that would otherwise escape.
![]()
UPDATE 20th February 2018:
The carrot seeds have now germinated (first noticed yesterday, 8 days after planting). One thing I’ll be watching out for here is whether the seedlings retain the seed case for a long time. I observed this for all seeds that sprouted in the previous experiments, and wonder whether it is in any way related to germinating in a high CO2 environment.
![]()
UPDATE 27th February 2018:
The seedlings are still growing, all 5 have germinated (2 inadvertently planted next to each other, one planted near the edge of the container). The photo below shows the seedlings under the purplish light that results from having four red LEDs and one blue:
![]()
UPDATE 11th March 2018:
Two of the seedlings died after a mouldy growth grew on them. Of the remaining three, one is near the edge of the bottle and doesn’t receive much light, one Has the seed holding the seed leaves closed, but the third now has its first true leaf (first noticed yesterday, 27 days after planting):
![]()
UPDATE 18th March 2018:
Yesterday I noticed that the seedling with the true leaf began to lean over to one side, and today all three remaining seedlings had fallen over completely.
![]()
![]()
My current belief is that this is due to the CO2 level reaching some threshold above which the seedlings rapidly die. I attached the CO2 sensor to the bottle and it read off scale (over 10000ppm), but I’m not sure exactly how high it was.
Out of curiousity I’ve left the lid off the bottle so see whether the seedlings are beyond the point of recovery.
One further thing I could try to reduce the rate at which CO2 levels increase in the bottle is to reduce the amount of organic material in the soil. Ideally I’d like to produce a soil that has a known quantity of organic material. I have the ingredients for making green sand (a mixture of bentonite clay and sand) for metal casting, so I could mix this with crushed up dead leaves. I could have a gradient in the amount of organic material in the soil, so that the top part of the soil that is initially the wettest doesn’t have any organic material.
-
Artificial lighting
02/04/2018 at 17:52 • 0 commentsIf and when I manage to grow carrots in a sealed bottle, I want to be able to do it largely independently of the external conditions. So I’ll need to control the temperature of the plant’s environment and the amount of light it receives.
I’ve set up a carrot seedling in a pot with three LEDs illuminating it. I used two red LEDs and one blue, having read that this proportion is supposed to be okay for plant growth.
![]()
I’m not sure exactly how much light energy the LEDs are emitting, but I estimate that the illuminated area around where the plant is growing is receiving no more than 25 W/m2. Will that be enough? Even though it looks bright in contrast with the room lighting, I think that it’s only about the same intensity as an overcast day.
UPDATE 15th February 2018:
The carrot seedling is now showing its first true leaf between the two seed leaves, as seen in the photo below.
![]()
I’ve been thinking about how to make more efficient use of the light that shines on a seedling. When the plant is small, most of the light from the LEDs is wasted, only a small fraction lands on the seedling. With an array of smaller LEDs, each directed to a small area of soil, it will be possible to illuminate an area closely matching the shape of the plant. It would be possible to adapt the shape of the illuminated area to the plant by using a camera to monitor the plant. But this seems overly complicated. Plants are already capable of adapting themselves to their circumstances, so instead of having a complex system of monitoring with a camera, I plan to have a fixed sequence of illumination patterns. Over the course of 2 or 3 months the pattern of illumination will grow in size - a few more LEDs will be turned on every few days - at a rate matched to the expected growth rate of the plant. Hopefully the plant will adapt itself to fit into the illuminated area.
UPDATE 11th March 2018:
The seedling has developed three true leaves now - two large ones that have been present for weeks, and one smaller one which appeared a few days ago. The stems are very elongated where the leaves grow upwards until they are almost touching the LEDs, so it seems as though they would prefer to have more light than this.
![]()
UPDATE 17th March 2018:
The third true leaf is already nearly as large as the other two. In the photo below you can see how two of the leaves have stretched right up to where the LEDs are to get as much light as possible. A fourth small true leaf has appeared, first noticed today. The main stem of the carrot, below where the leaves split off, is very thin and could not support the plant - the plant is held in place partly by the leaf in contact with the cardboard.
![]()
UPDATE 13th April 2018:
Earlier on where I wrote "no more than 25 W/m2", I wasn't sure how efficient the LEDs were. I've since estimated the efficiency of the LEDs (see log entry 'More on artificial lighting'), and I think the actual figure is about 7 W/m2, so a lot less light than the plant would receive outdoors. -
Source code for using the MH-Z14A CO2 sensor
01/28/2018 at 21:28 • 0 commentsI've put my source code for using the CO2 sensor (on Linux) here:
https://github.com/WillStevens/co2_sensor
It makes use of a command for turning off 'Automatic Baseline Correction', and also a command for setting the range of the sensor. Both commands can be found in the Chinese version of the documentation for the sensor, here:
-
Some useful references
01/28/2018 at 19:59 • 0 commentsI found out a few things about terraria, and about the effect of high levels of CO2 on plants in the following places:
- N. Schwarz & B. R. Strain (1990) Carbon — A plant nutrient, deficiency and sufficiency, Journal of Plant Nutrition, 13:9, 1073-1078, DOI: 10.1080/01904169009364136
This paper discusses the effect of exposing various vegetables to a CO2 concentration of 10000ppm (1%) for 6 days. Normal outdoor concentration is about 400ppm at the time of writing. Generally, the plants exhibited observable effects, and growth was delayed, but the plants recovered after the CO2 concentration was returned to normal levels. Out of curiosity I looked up what the maximum CO2 concentration is in spacecraft, and found here that it's usually about 0.5%, but that people can tolerate at least 2% for days with no obvious harmful effects. - Stephen L. Thompson (2007) Inquiry in the Life Sciences: The Plant-in-a-Jar as a Catalyst for Learning, Science Activities: Classroom Projects and Curriculum Ideas, 43:4, 27-33, DOI: 10.3200/SATS.43.4.27-33
This paper discusses the educational value of growing a plant in a sealed jar. The activity prompts students to think about what plants need to grow and where they get it from, and how to answer those questions. This paper also mentions Nathanial Bagshaw Ward, who was one of the first people to grow plants in sealed containers. His work led to the use of sealed containers for transplanting plants on ships. - Martin et al. Extreme Physiology & Medicine 2012, 1:4. A paradigm of fragile Earth in Priestley's bell jar.
This paper describes an experiment about sealing a person in a container for 48 hours with enough plants to produce the oxygen that he needed. - Marsarium 9
A fern was grown for 30 days in soil thought to be similar to that found on Mars, in an atmosphere with the same composition as on Mars (albeit at 1 bar of pressure rather than the 6 mbar pressure found on Mars). The Fern in this project was apparently able to survive in a 96% CO2 environment for 30 days. The atmosphere wasn't monitored though, so I don't know how the atmospheric composition changed over time. Given the paper mentioned above about harmful effects of 1% CO2 concentration on plants, it would be interesting to know whether the CO2 level stayed at 96% for the whole 30 days, or whether the fern converted some of it to O2, or whether any of it leaked out.
UPDATE 23rd April 2018:
I found this guide to plant lighting very informative:
https://horticulture.ahdb.org.uk/sites/default/files/u3089/Lighting_The-principles.pdf
This review of growing plants in space was also interesting, although the problems of growing plants in a weightless environment (such as preventing pooling and maintaining airflow) are somewhat different from the problems of growing plants on Mars:
https://www.sciencedirect.com/science/article/pii/S2214552415300092?via%3Dihub
- N. Schwarz & B. R. Strain (1990) Carbon — A plant nutrient, deficiency and sufficiency, Journal of Plant Nutrition, 13:9, 1073-1078, DOI: 10.1080/01904169009364136
-
Soil and CO2
01/06/2018 at 00:58 • 0 commentsI want to test a hypothesis that if I start with initially dry soil, then add a small quantity of water (and some microbes on a small piece of damp soil), I will end up with an escalating production of CO2: the small quantity of water will allow some microbes to consume organic material in the soil, producing CO2 and water. The additional water will make more of the soil damp, allowing the microbes to spread and consume more organic material, until eventually all of the soil is damp.
Based on observing the experiment described in the “Second Attempt” log entry, in which I started with 36ml of water and most of the lower part of the soil was obviously dry to begin with, and the very lowest part still looks dry now (4 weeks later), I think that this process will take several weeks to run.
To begin with, I want to check that completely dry soil does not release CO2. I took 325g of dry soil and placed it in a bottle with the CO2 sensor attached. The CO2 level began at about 1100ppm. Over the course of 3.5 hours I was very surprised to see the CO2 level decrease to 850ppm. What could be the reason for this? One possibility that sprang to mind was that the apparatus had a leak, and the CO2 level in the room was decreasing. To test this I removed the CO2 sensor from the bottle at the end of the experiment and it quickly went back up to over 1000ppm, so a leak can be ruled out as the explanation.
Another possible explanation is that the dry soil had come from a radiator, so it was warm (I estimate no more than 40C) when it went into the bottle. Is it possible that as the soil cools it absorbs CO2 from the air? Or could the temperature or pressure be affecting the sensor reading? Both of these seem plausible: I have read that some minerals adsorb gases when cooled and release them when heated (this is how adsorption pumps work - these have been proposed as a highly reliable way of compressing the atmosphere of Mars for use as a CO2 supply using few moving parts and with focused solar heating as the energy source); the CO2 sensor does not claim to a be highly accurate, and is influenced by environmental factors.
UPDATE 8th January 2018:
After a couple of days the CO2 level in the bottle containing dry soil was down to 650ppm. I decided to add the water (12 ml) and watch what happened to the CO2 level. I had expected that the CO2 level would increase gradually over the next few weeks. To my surprise there was a sudden increase over the next few minutes - it went up to 1600ppm quickly, then climbed more slowly. 9 hours later it was at 2300ppm. It is as though adding the water triggered an immediate release of CO2 from the soil. Could it be that gas adsorbed onto soil particles is released when it gets wet?
If my interpretation of these experiments is correct (I’m not sure yet whether it is), then it means that I can greatly reduce the initial level of CO2 in a sealed bottle by setting up the bottle outside (where the CO2 level is lower than inside), by adding the soil warm and keeping it warm for about 30 minutes, and by waiting for about 30 minutes after I add water to the soil before closing the bottle, so that the initial release of CO2 from the soil escapes from the bottle.
UPDATE 13th January 2018:
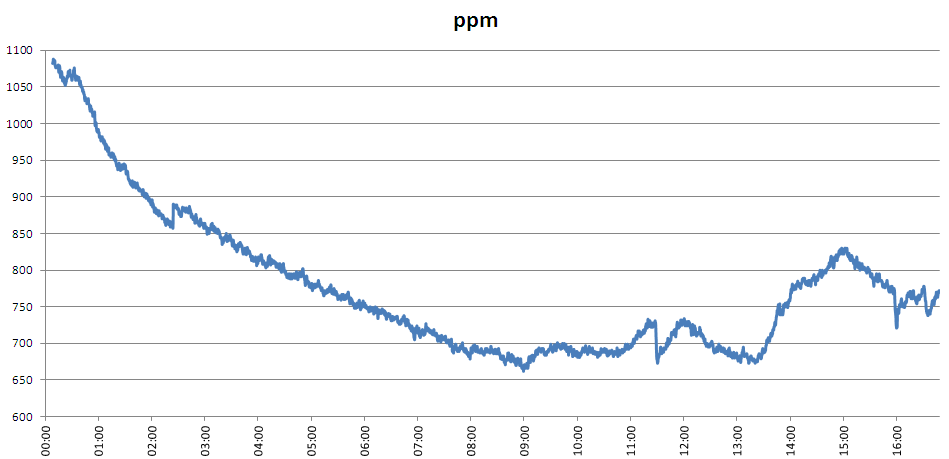
My attempt to measure the change in CO2 level in a sealed bottle containing dry soil with 12ml of water and some soil microbes added is hampered by three issues. Firstly, I haven't yet checked my apparatus for leaks - I haven't seen any results which make me think it leaks, but I'm not certain because I haven't explicitly tested this. Secondly, I've found that there is a daily variation in CO2 level in the bottle which I believe is correlated with the temperature in the room. This can be seen in the graph below:
![]()
I turn my heating off when I go to bed - shortly before midnight last night - and the CO2 level began to drop shortly afterwards. At weekends I turn my heating on in the morning - shortly after 10am this morning - which is when the CO2 level began to rise. I believe that the amount of CO2 adsorbed by the soil is temperature dependent. When the soil warms it gives off CO2, when it cools it adsorbs again.
Thirdly, the CO2 sensor has a feature called Automatic Baseline Correction (ABC). Some of the documentation for the MH-Z14A says: "...sensor itself do zero point judgement and automatic calibration procedure intelligently after a continuous operation period. The automatic calibration cycle is every 24 hours after powered on". The documentation says that the ABC function is not suitable for greenhouses and that I should shut down the automatic calibration function. The English translation of the documentation doesn't explain how to do this, so I've deliberately not left the sensor powered on for 24 hours so that ABC does not happen. I believe that the idea behind ABC is that if the sensor is used in a situation where the CO2 level regularly goes down to the outdoor CO2 level (which is about 400ppm), the sensor will automatically calibrate itself against this, by looking for the lowest reading over the course of a day. There is some discussion of the feature in a slightly different product (the MH-Z19) here: https://github.com/letscontrolit/ESPEasy/issues/466
Today I found out that the MH-Z19 has commands to turn ABC on and off, they can be found in this file from the ESPEasy project on GitHub:
https://github.com/TD-er/ESPEasy/blob/mega/src/_P049_MHZ19.ino
Then I discovered the Chinese documentation for the MH-Z14A here:
http://style.winsensor.com/pro_pdf/MH-Z14A.pdf
and confirmed with the help of google translate that the same command (command 0x79) works with the MH-Z14A.
The following sequence of bytes turns ABC off:
0xFF 0x01 0x79 0x00 0x00 0x00 0x00 0x00 86
and this command turns it on:
0xFF 0x01 0x79 0xA0 0x00 0x00 0x00 0x00 E6
I'm going to issue the 'ABC off' command whenever I use the sensor. I'm going to stop this current experiment and connect the CO2 sensor to a bottle containing no soil and monitor it for a day - I would expect the CO2 level to remain constant. This will help to check whether or not the apparatus leaks, and whether the variation in CO2 level shown in the graph above really is down to adsorption and release from the soil. Once I've done this, I'll redo this experiment and leave the sensor connected for a longer period.
UPDATE 20th January 2018:
I ran the sensor overnight attached to an empty bottle, and the CO2 level inside went down overnight (see graph below).
![]()
To be sure that the reading wasn't being affected by temperature, I made a note of what the CO2 level was at a temperature of 16.1C in the evening (1064 ppm), then looked again in the morning when the temperature had risen back up to 16.1C - it was 700ppm, so the apparatus had a leak.
A simpler way of testing for a leak that didn't occur to me up until this point was to put the apparatus upside-down in a jug of water while gently squeezing the bottle, being careful not to release my grip and accidentally suck water into the CO2 sensor.
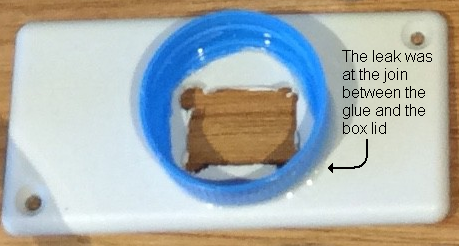
I found a small hole in the glue joint where the bottle lid was glued onto the plastic box that holds the sensor (see photo).
![]()
The epoxy glue that I am using doesn't stick very well to the plastic box (or the bottle lid), and there was a crack beneath the glue where the glue meets the plastic box. I suspect that the action of screwing and unscrewing the sensor onto bottles - which requires applying pressure to the sides of the bottle lid and flexing it a little - had caused the crack. After drying everything thoroughly, I removed the lid from the box, and removed the old glue.
Before applying more glue, to help the glue hold more strongly, I roughened the plastic box lid very thoroughly with sand-paper - I had already done this before, but I made sure to make it even rougher this time. I also roughened round the edge of the bottle lid where it attaches to the box lid. I used more glue this time. I think that a more flexible glue, and a glue that adheres better to plastic, would be better than the epoxy glue I'm using.
Because this leak affected some of the experiments I've done, it affects my interpretation of some of the results. I'm no longer sure about the idea that soil adsorbs and releases CO2 as the temperature changes. Changes in temperature in the room that the experiment was running in are also correlated with changes in CO2 concentration - in the day when I'm in the room I have the heating on, and also exhale CO2. Overnight the heating is off and at the same time the CO2 level gradually drops because I'm not in the room.
I leak tested the re-glued apparatus first in the water jug, then overnight with a bottle containing an elevated level of CO2. It seemed fine - the CO2 level remained constant overnight (see graph below). I believe that the initial drop from 1400ppm to 1270ppm that occurs before 00:00 hours is because the CO2 sensor had previously been in a higher concentration environment, so it took time to reach equilibrium with the bottle. Since any leak rate is proportional to the difference in CO2 concentration between the inside and the outside of the bottle, it would be sensible to redo this leak test at a much higher CO2 concentration.
![]()
-
Third attempt
01/04/2018 at 23:31 • 0 commentsThis experiment was started on 2nd January 2018. It is the same as the second experiment (started 8th December 2017), but with only 12ml of water added to the bottle rather than 36ml. I also tried not to powder the soil too finely when I added it to the bottle.
I hope that because the soil is drier initially, CO2 will be released more slowly from the soil into the air, and the seedlings will last longer.
The progress of this attempt will be added as updates to this log entry.
UPDATE 17th January 2018:
The carrot seeds have begun to sprout (to the right of the centre in the photo below). Three weed seeds that were present on the moss have also begun to sprout (to the left In the photo).
![]()
UPDATE 24th January 2018:
One of the carrot seedlings died off. Furry growth developed around its leaves, and it collapsed. The larger of the seedlings is still doing well, and a third has sprouted and is unfurling itself (behind the weeds in the photo below). The weed seedlings are thriving, one of them has the beginnings of a second pair of leaves.
I’ve noticed in this and previous experiments that the carrot seed coat clings onto the first pair of leaves of the seedlings.
![]() UPDATE 28th January 2018:
UPDATE 28th January 2018:This experiment has now been running for 26 days and the largest carrot seedling still looks healthy. It has exceeded the time by which the seedlings in the 'Second Attempt' experiment had collapsed.
UPDATE 3rd February 2018:
The number of molecules of gas in the bottle has decreased. The bottle is permanently indented because of the reduction of pressure inside:
![]()
Could this be because some of the nitrogen in the bottle has been fixed by soil microbes?
The carrot seedlings remains alive, but doen’t seem to be growing very much. The taller seedling still has the seed case stuck to the leaves, holding them together and preventing them from opening out. One of the carrot seedlings hasn’t straightened up, I wonder whether this is because it also has it’s leaves stuck to the seed case, which might be stuck to something in the soil. The weeds are looking fine, and the third leaf on each has grown to about half the length of the larger leaves.
![]()
UPDATE 7th February 2018:
I’m still puzzling over the loss of gas volume inside this bottle described above. I measured the loss of volume by immersing the bottle in a jug and comparing the displacement with a bottle full of air. The difference was about 50cc. So 50cc of gas has either escaped from the bottle, or been used up in some biological or chemical or surface process.
I believe that both photosynthesis, respiration and nitrogen fixation result in no net change in the number of gas molecules. In photosynthesis CO2 is replaced with O2, in respiration O2 is replaced with CO2. In nitrogen fixation N2 is replaced with H2. Is there some other process happening that I’m not aware of? Is it true that the number of gas molecules is unchanged by these proceses? (My understanding of these things might be oversimplified).
I read that gases diffuse through PET slowly over time, so if the partial pressure of a gas is larger inside the bottle than outside, then that gas could leak out, leading to a decrease in the number of molecules of that gas inside the bottle. I guess that H2 would leak quite quickly, so if nitrogen fixation is taking place, and if this is producing H2, then that could be an explanation. But has there been enough nitrogen fixation to produce 50cc of H2?
I’m fairly sure that the partial pressure of CO2 in the bottle is higher than the outside, so that will slowly diffuse out. But should it really happen that quickly? Even if all the O2 in the bottle were replaced with CO2 (which I don’t believe is the case), then the partial pressure difference of CO2 between inside and outside would be about 0.2 bar. And wouldn’t O2 leak in at the same time that CO2 leaked out? Or does CO2 diffuse more quickly through PET than O2?
It should be possible to test questions about approximate expected rates of diffusion of H2 and CO2 through PET with separate experiments beginning with bottles filled with these gases and nothing else.
This project is generating a lot of new questions that I didn’t have at the outset, so it’s becoming more interesting as it progresses!
UPDATE 20th February 2018:
Having read a bit more about it, I’m fairly sure that the reason that the bottle has lost some of its air molecules is that CO2 has escaped through the PET bottle. I had wondered whether the loss might have been due to nitrogen fixation and subsequent loss of hydrogen, but I don’t believe that nitrogen fixation inside the bottle is happening at a fast enough rate for this to be the cause.
A large amount of the oxygen in the bottle will have been converted to CO2 through soil respiration, and I’ve learned that CO2 passes through PET much more readily than N2, O2 or H2. At first it seems counterintuitive that gas can travel against the total pressure gradient, but it’s the partial pressure that counts: because the partial pressure of CO2 inside the bottle is higher than outside, CO2 molecules will slowly leak out of the bottle, lowering the total pressure inside the bottle and causing it to contract.
I measured a loss of 50cc, and since the bottle had about 300cc of air in it initially, of which about 60cc was O2, this means that most of the O2 has been used up and replaced with CO2 during soil respiration, then leaked out of the bottle.
It occurred to me that a material that allows CO2 to pass through more readily than O2 could be used to extract CO2 from the Martian atmosphere into a greenhouse without using a pump. CO2 makes up about 96% of the atmosphere of Mars, at a partial pressure of about 6 mbar (and probably upto 12 mbar in the lowest lying parts of Mars). Plants grow on Earth with a CO2 partial pressure of 0.4 mbar. A greenhouse with walls made from a semipermeable barrier like PET (but capable of withstanding the higher radiation levels found on Mars - see note below) would draw in CO2 from the atmosphere as plants use it up during photosynthesis. The pressure in the greenhouse would increase because the O2 that the plants produce would leak out more slowly than the CO2 leaked in. The maximum pressure that it would reach would depend on the ratio of the rate at which O2 and CO2 permeate the barrier material. If O2 passes through the barrier 10 times more slowly than CO2, then such a self-pressurising greenhouse could reach 120 mbar in the lowest lying area of Mars.
This probably isn’t a new idea, so if anybody knows of anything already written about this please add a link in a comment. Also, if you think it won’t work, please add a comment.
Note that the transparent part of the greenhouse could be made from a more robust material such as glass, and the semipermeable barrier could be in an underground tunnel to protect it from radiation present on the surface.
-
Measuring CO2 levels
01/02/2018 at 23:11 • 0 commentsWhat happens to the mix of gases inside a sealed bottle with a plant growing in it? My current understanding of the gas exchange processes that take place inside the bottle is as follows:
Microbes in the soil consume dead organic material in the soil. They use oxygen from the air and produce CO2 and water from the organic material.
Some microbes in the soil fix nitrogen from the air - I don’t know much about this yet, and don’t know whether nitrogen levels in the air will be a factor that I need to consider over the timescales that I’m interested in (months).
During photosynthesis, plants in the bottle (moss and the carrot seedlings), take CO2 from the air and release oxygen (this also uses up water). The plants also respire - taking in oxygen and releasing CO2 and water. But so long as the plant is growing the net effect is to remove CO2 from the air and produce oxygen.
In the log entry “First Attempt” I noted that nothing germinated in two bottles that I set up at the beginning of November, and all weeds that sprouted subsequently died. I wondered whether this was due to the soil producing more CO2 than the plants could consume.
I used an MHZ-14A CO2 sensor to measure the CO2 in one of the bottles described in the “First Attempt” log entry. Although this meant removing the cap of the bottle in order to attach the sensor, I thought that not much CO2 would escape when I did this.
The MHZ-14A seems easy to use. The one that I ordered didn’t come with documentation, but I found some here and here. It has a UART with 5V TTL compatible IO, and has a command for directly returning the CO2 level in parts-per-million (ppm), so it is straightforward to hook it up to a laptop and display the CO2 level on the screen.
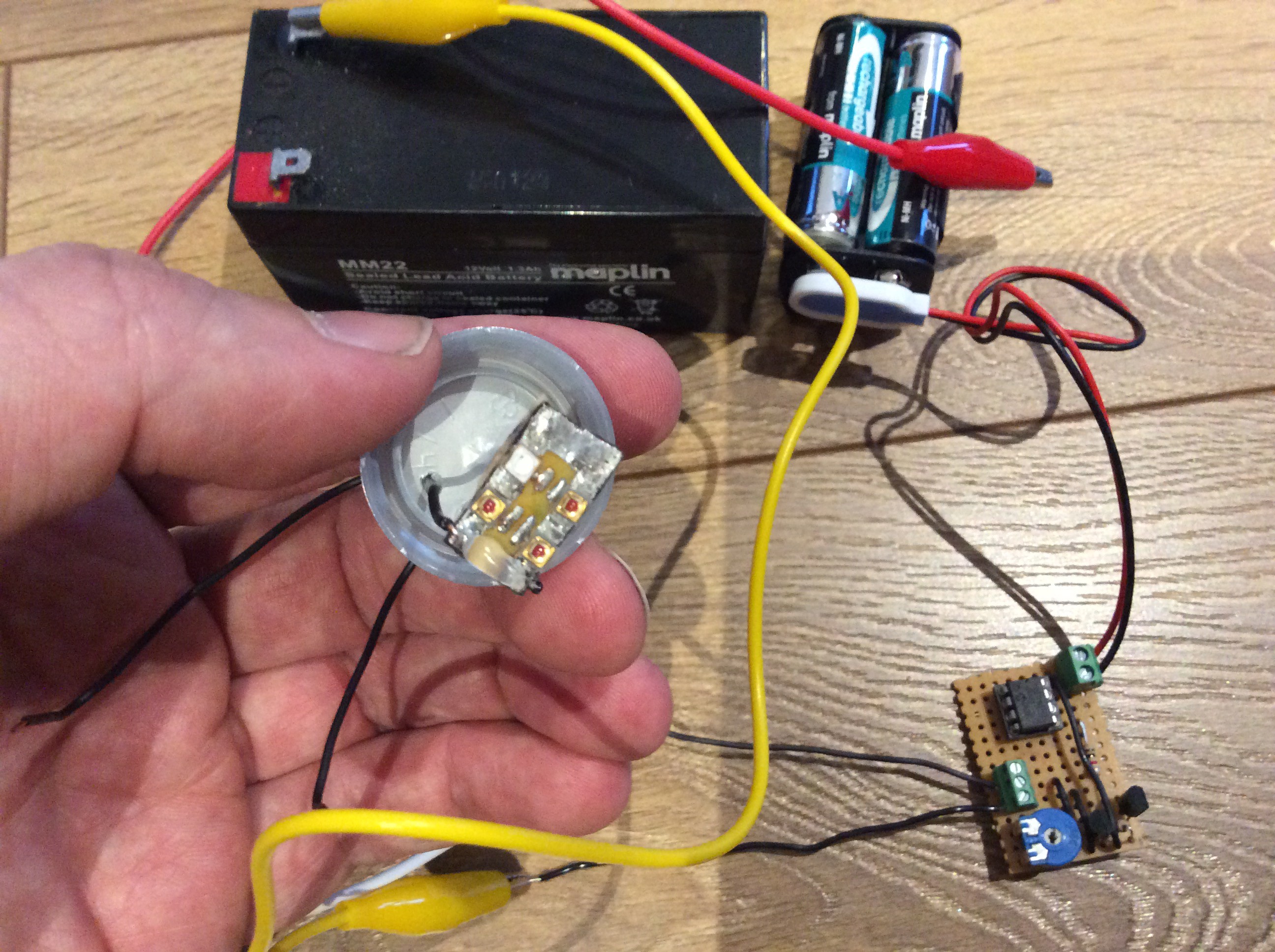
I have the sensor mounted in an enclosure with a bottle lid glued onto it so that I can easily screw it onto the top of a bottle to check the CO2 level in the bottle. The photos below shows the sensor and its enclosure, and the sensor attached to a bottle (with a MAX232 circuit attached so that it can be connected to a laptop).
![]()
![]()
I noticed that the CO2 level in my room was 1300ppm. When I opened the door to let in fresh air it went down to 750ppm in about 15 minutes. When I breathed into a bottle and attached the CO2 sensor it reported 5000ppm, indicating that the concentration was at or above the measurable range. I haven’t calibrated the sensors, but these levels seem reasonable.
The CO2 reading in one of the bottles described in the “First Attempt” log entry was greater than or equal to 5000ppm, so this is perhaps the reason why everything died.
How can CO2 production from the soil be slowed down? One way is to use less soil, another is to put enough plants in the bottle to absorb the CO2 produced, another strategy that might work is to limit the amount of water - perhaps drier soil will release less CO2. Limiting the water might not hamper plant growth because more water will be produced (from organic matter) by soil microbes at the same time that they produce CO2.
Growing vegetables in sealed containers
I want to work out whether or not it is possible to grow vegetables indoors in sealed, airtight containers.
 will.stevens
will.stevens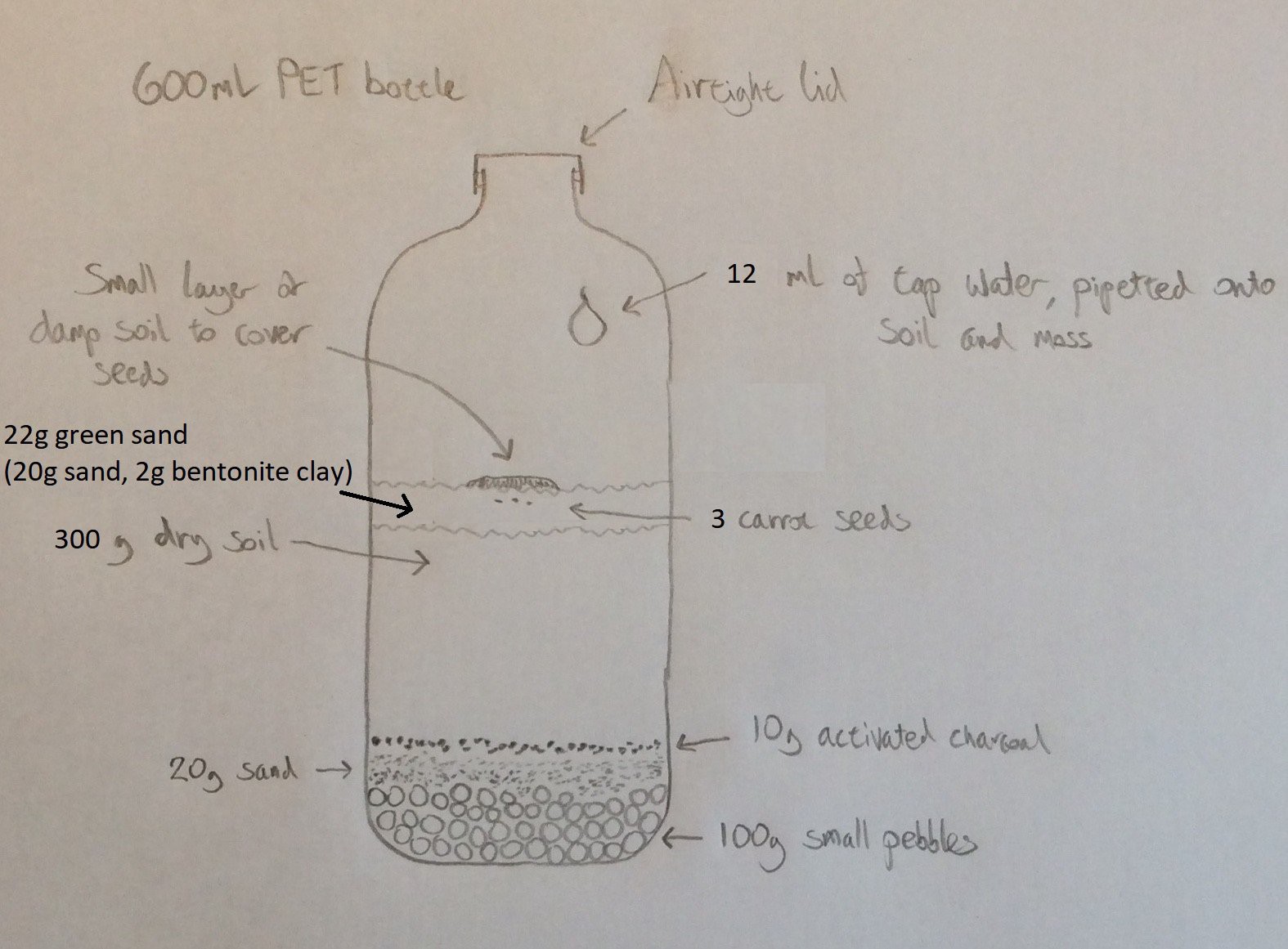























 UPDATE 28th January 2018:
UPDATE 28th January 2018:


