The basic idea
In common syntheses of aspirin, salicylic acid is treated with acetic anhydride, causing a chemical reaction that turns salicylic acid's hydroxyl group into an ester group. Because acetic anhydride is used for the synthesis of heroin by the diacetylation of morphine, acetic anhydride is listed as a U.S. DEA List II precursor, and therefore it is restricted in many other countries including mine, so I won't use it. Instead I'll follow a slightly different path as a starting point:
As acetic acid is less reactive than acetic anhydride, the reaction yield will be lower. Furthermore the purity level of the acetyl-salicylic acid will be lower.
Balanced equation:
Molar mass salicylic acid: 138.122 g/mol
Molar mass acetic acid: 60.052 g/mol
Molar mass acetyl-salicylic acid: 180.157 g/mol
Molar mass water: 18.015 g/mol
Theoretical yield:
138.122 g salicylic acid + 60.052 g acetic acid → 180.157 g acetyl-salicylic acid
But I expect the actual yield will be only half of the theoretical one.
Synthesis of aspirin at a laboratory scale
1.
- Measure 1 g of salicylic acid (
± 0.01 g)


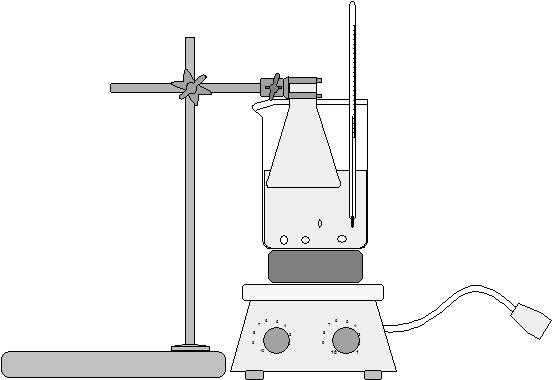
- Place it under fume hood into 50-ml Erlenmeyer flask
- Cover salicylic acid crystals with 2 - 2.5 ml glacial acetic acid


- Swirl flask and add 2.5 drops of concentrated sulfuric acid

- Heat flask gently in a 70°C hot water bath for 10 minutes

2.
- Remove flask from water bath, add 15 ml distilled ice water
- Allow mixture to stand for 5 minutes
- Chill solution in an ice bath until crystallization of aspirin occurs
- Stir occasionally
- If crystallization does not occur, scratch walls and bottom of flask with a glass stirring rod to promote crystallization
- If an oil appears instead of a solid, re-heat flask in hot water bath until oil disappears and cool again
3.
- Set up a vacuum filtration apparatus with Hirsch funnel

- Transfer solid aspirin onto filter paper
- Add small amounts of distilled ice water and repeat until transfer of aspirin crystals is complete
- Wash aspirin crystals on filter paper with 10 ml of distilled ice water
- Pour filtrate in the sink
4.
- Transfer aspirin crystals from filter paper to a 100-ml beaker
- Dissolve crystals in a minimum volume of 10 ml pure ethanol

- Warm solution in a 60°C hot water bath
- Pour 25 ml distilled water into solution after solution has reached 60°C
- If a solid forms, continue warming until solid dissolves
5.
- Remove beaker from heat
- Cover beaker with a watch-glass
- Set beaker aside to cool slowly. Do not disturb solution
- Needle-like crystals of pure aspirin should form overnight
Testing for Impurities
Iron(III) chloride can be used to determine the purity of the synthesized aspirin. Iron(III) chloride forms a purple complex with the phenol group. Salicylic acid is a phenol. It cannot be removed by using cold distilled water as its solubility in water is poor. So if salicylic acid is still present, the product will turn purple when Iron(III) chloride is added.
Synthesis of salicylic acid at a laboratory scale
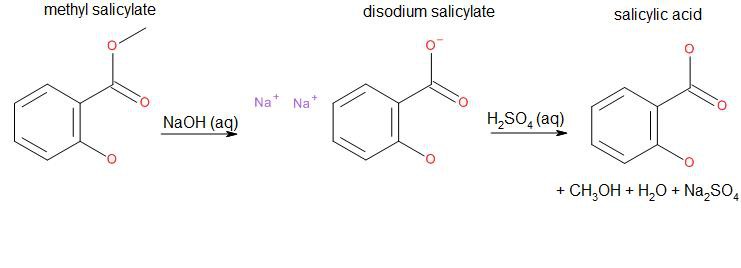
Salicylic acid can be synthesized by a base-catalyzed hydrolysis of methyl salicylate, commonly known as oil of wintergreen or wintergreen oil:
Balanced equation:As we can see, side products are water, sodium sulfate and methanol. The hydrate of sodium sulfate is known as Glauber's Salt, a formerly used general purpose laxative. Methanol is the simplest alcohol and highly toxic.
Molar mass methyl salicylate: 152.15 g/mol
Molar mass sodium hydroxide: 39.9971 g/mol
Molar mass sulfuric acid: 98.079 g/mol
Molar mass sodium sulfate: 142.04 g/mol
Molar mass methanol: 32.04 g/mol
Therefore theoretically:
152.15 g methyl salicylate + 79.9942 g sodium hydroxide + 98.079 g sulfuric acid → 138.122 g salicylic acid + 142.04 g sodium sulfate + 32.04 g methanol + 18.015 g water
Lab synthesis procedure:
1.
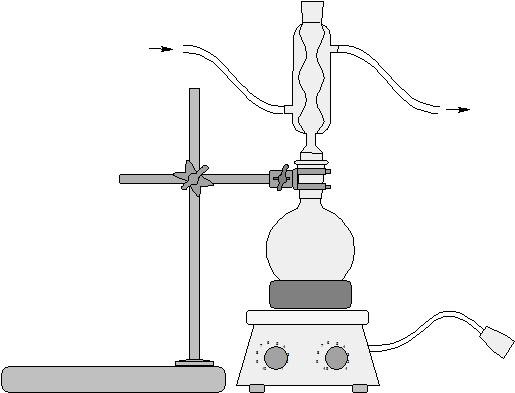
- Set up water jacketed condenser apparatus with 50 ml round bottom flask

- Pour 3.5 ml of distilled water into the flask
- Add 0.448 g sodium hydroxide

- Swirl until solid dissolved
- Add 0.23 ml of methyl salicylate to the NaOH solution

- Drop boiling stone to bottom of flask
- Attach...
 M. Bindhammer
M. Bindhammer

















 Theodor Hillebrand
Theodor Hillebrand
 MechaTweak
MechaTweak
 sgall17a
sgall17a
 Morning.Star
Morning.Star
This project made me research a little and since there's not much going on here right now let me take the liberty to elaborate.
It seems most likely that where simple meds like Aspirin aren't available, proper lab conditions and reagents of sufficient quality are also not available. So as long as you can grow willows - which are fairly robust - and get powdered silica it is possible to brew a watery extract of willow bark (which has around 1% salicin) and extract the salicin by column chromatography (required to get rid of the tannins).
Salicin will be metabolized to salicylic acid by gut microbes and that's it. It lacks the boost in efficacy caused by the acetyl group but that's as sustainable as is possibly gets with growing shrubs and filtering tea through ground rocks.
Now if we could only find a nifty way to get that acetylation going. Some fungus or bacterium that can pull of acetyl transferase action perhaps? Sadly not really my field of expertise.
---------------------
https://bioweb.uwlax.edu/BIO203/2011/girard_stev/habitat.htm
Periodic Videos on youtube: v=amTAuK25P6c