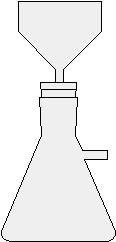
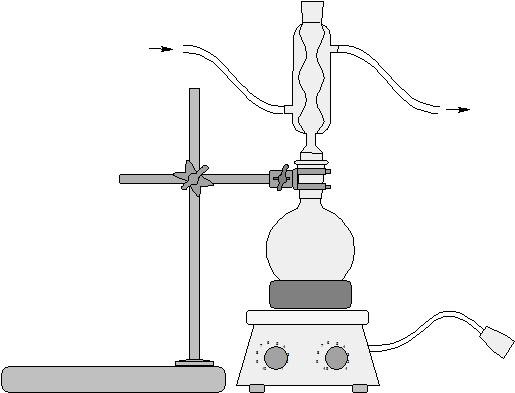
The basic idea
In common syntheses of aspirin, salicylic acid is treated with acetic anhydride, causing a chemical reaction that turns salicylic acid's hydroxyl group into an ester group. Because acetic anhydride is used for the synthesis of heroin by the diacetylation of morphine, acetic anhydride is listed as a U.S. DEA List II precursor, and therefore it is restricted in many other countries including mine, so I won't use it. Instead I'll follow a slightly different path as a starting point:
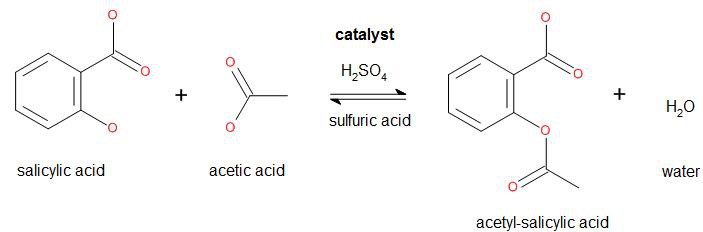
As acetic acid is less reactive than acetic anhydride, the reaction yield will be lower. Furthermore the purity level of the acetyl-salicylic acid will be lower.
Balanced equation:
Molar mass salicylic acid: 138.122 g/mol
Molar mass acetic acid: 60.052 g/mol
Molar mass acetyl-salicylic acid: 180.157 g/mol
Molar mass water: 18.015 g/mol
Theoretical yield:
138.122 g salicylic acid + 60.052 g acetic acid → 180.157 g acetyl-salicylic acid
But I expect the actual yield will be only half of the theoretical one.
Synthesis of aspirin at a laboratory scale
1.
- Measure 1 g of salicylic acid (
± 0.01 g)
![]()
![]()
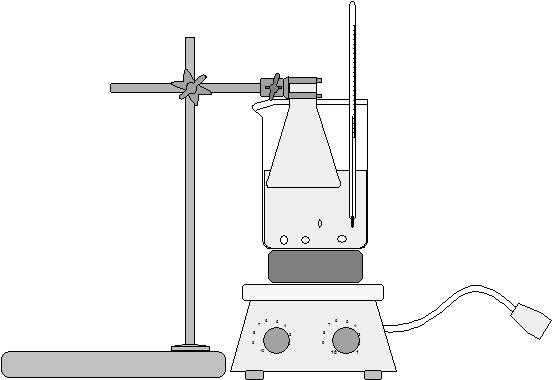
- Place it under fume hood into 50-ml Erlenmeyer flask
- Cover salicylic acid crystals with 2 - 2.5 ml glacial acetic acid
![]()
![]()
- Swirl flask and add 2.5 drops of concentrated sulfuric acid
![]()
- Heat flask gently in a 70°C hot water bath for 10 minutes
![]()
2.
- Remove flask from water bath, add 15 ml distilled ice water
- Allow mixture to stand for 5 minutes
- Chill solution in an ice bath until crystallization of aspirin occurs
- Stir occasionally
- If crystallization does not occur, scratch walls and bottom of flask with a glass stirring rod to promote crystallization
- If an oil appears instead of a solid, re-heat flask in hot water bath until oil disappears and cool again
3.
- Set up a vacuum filtration apparatus with Hirsch funnel
![]()
- Transfer solid aspirin onto filter paper
- Add small amounts of distilled ice water and repeat until transfer of aspirin crystals is complete
- Wash aspirin crystals on filter paper with 10 ml of distilled ice water
- Pour filtrate in the sink
4.
- Transfer aspirin crystals from filter paper to a 100-ml beaker
- Dissolve crystals in a minimum volume of 10 ml pure ethanol
![]()
- Warm solution in a 60°C hot water bath
- Pour 25 ml distilled water into solution after solution has reached 60°C
- If a solid forms, continue warming until solid dissolves
5.
- Remove beaker from heat
- Cover beaker with a watch-glass
- Set beaker aside to cool slowly. Do not disturb solution
- Needle-like crystals of pure aspirin should form overnight
Testing for Impurities
Iron(III) chloride can be used to determine the purity of the synthesized aspirin. Iron(III) chloride forms a purple complex with the phenol group. Salicylic acid is a phenol. It cannot be removed by using cold distilled water as its solubility in water is poor. So if salicylic acid is still present, the product will turn purple when Iron(III) chloride is added.
Synthesis of salicylic acid at a laboratory scale
Salicylic acid can be synthesized by a base-catalyzed hydrolysis of methyl salicylate, commonly known as oil of wintergreen or wintergreen oil:
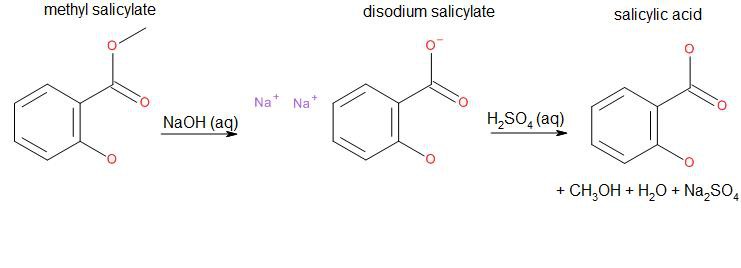
As we can see, side products are water, sodium sulfate and methanol. The hydrate of sodium sulfate is known as Glauber's Salt, a formerly used general purpose laxative. Methanol is the simplest alcohol and highly toxic.
Molar mass methyl salicylate: 152.15 g/mol
Molar mass sodium hydroxide: 39.9971 g/mol
Molar mass sulfuric acid: 98.079 g/mol
Molar mass sodium sulfate: 142.04 g/mol
Molar mass methanol: 32.04 g/mol
Therefore theoretically:
152.15 g methyl salicylate + 79.9942 g sodium hydroxide + 98.079 g sulfuric acid → 138.122 g salicylic acid + 142.04 g sodium sulfate + 32.04 g methanol + 18.015 g water
Lab synthesis procedure:
1.
- Set up water jacketed condenser apparatus with 50 ml round bottom flask
![]()
- Pour 3.5 ml of distilled water into the flask
- Add 0.448 g sodium hydroxide
![]()
- Swirl until solid dissolved
- Add 0.23 ml of methyl salicylate to the NaOH solution
![]()
- Drop boiling stone to bottom of flask
- Attach flask to water jacketed condenser
- Heat mixture for 15 minutes under fume hood
2.
- After reflux, separate heat source, allow equipment to cool down to room temperature
- Remove condenser
- Add 3 M sulfuric acid in 0.5 ml increments till white precipitate forms and stays
![]()
- Add another 0.5 ml sulfuric acid
3.
- Set up a vacuum filtration apparatus with Hirsch funnel
![]()
- Filter mixture
- Dissolve salicylic acid crystals by using a minimal amount of boiling hot distilled water
- Let the solution cool to room temperature, then cool it in ice bath for 8 minutes
- Vacuum filter again and let product dry
Aspirin lab-on-a-chip
A lab-on-a-chip (LOC) is a device that integrates one or several laboratory functions on a single chip of only millimeters to a few square centimeters in size.
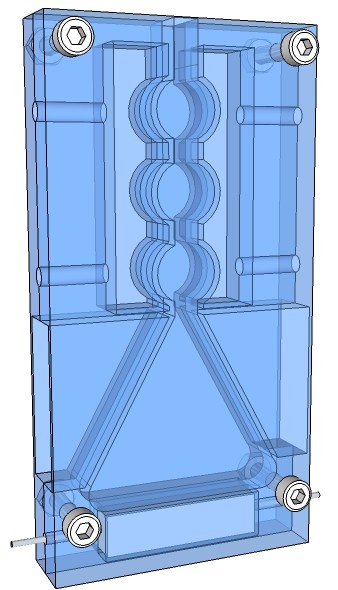
The rendering below shows a jacketed condenser apparatus, put together in sandwich construction, milled out from two borosilicate glass plates. The heating element is just a 1.5 Ω 50W electrical resistor as it is used in hot beds of 3-D printer.
Another possibility would be to 3-D print structure of apparatus with fire-proof and chemical resistant silicone on carrier glass plates:
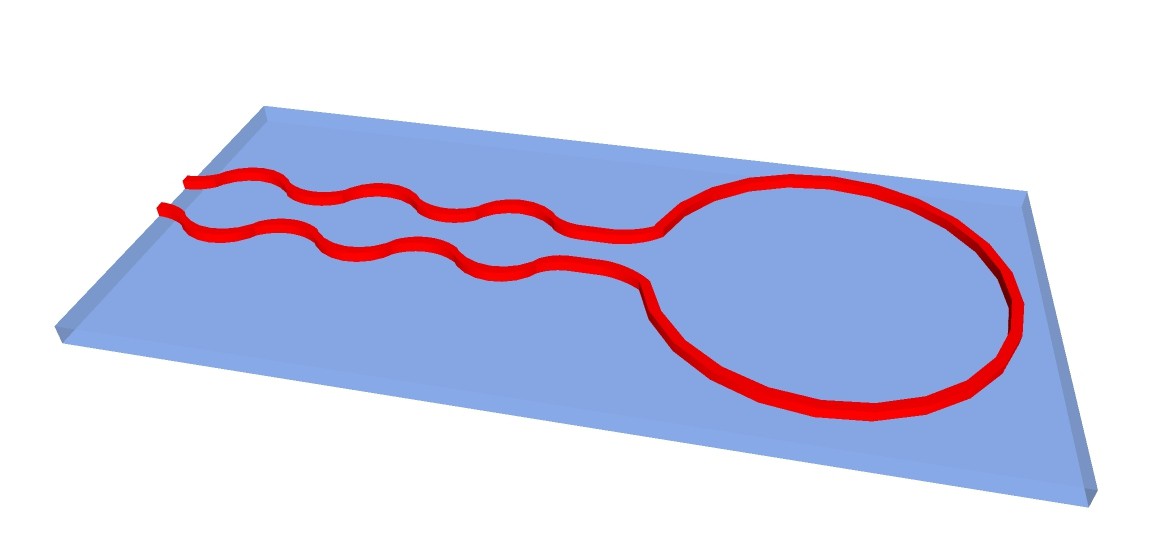
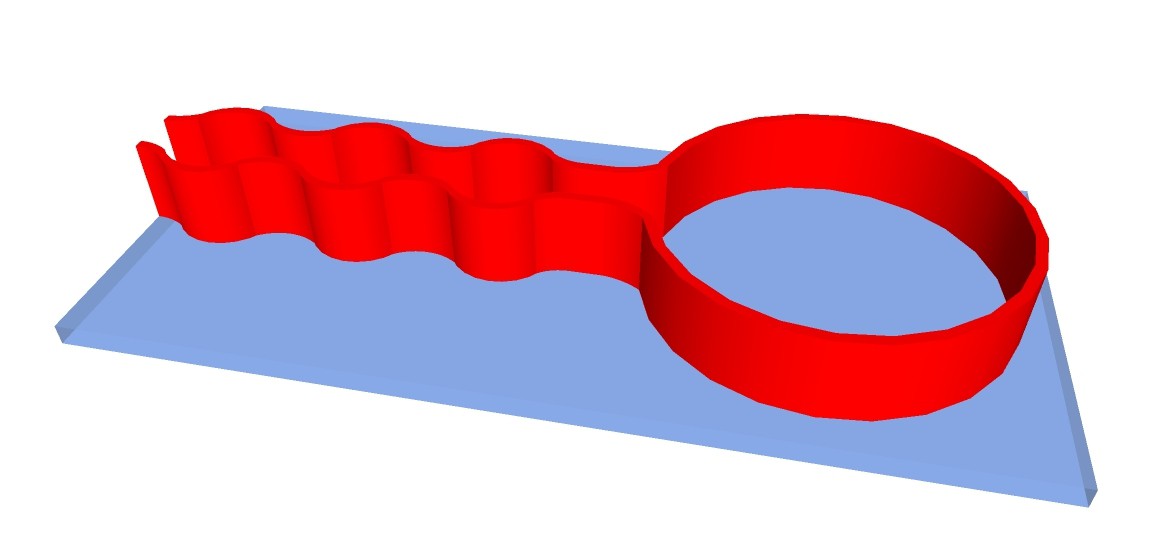
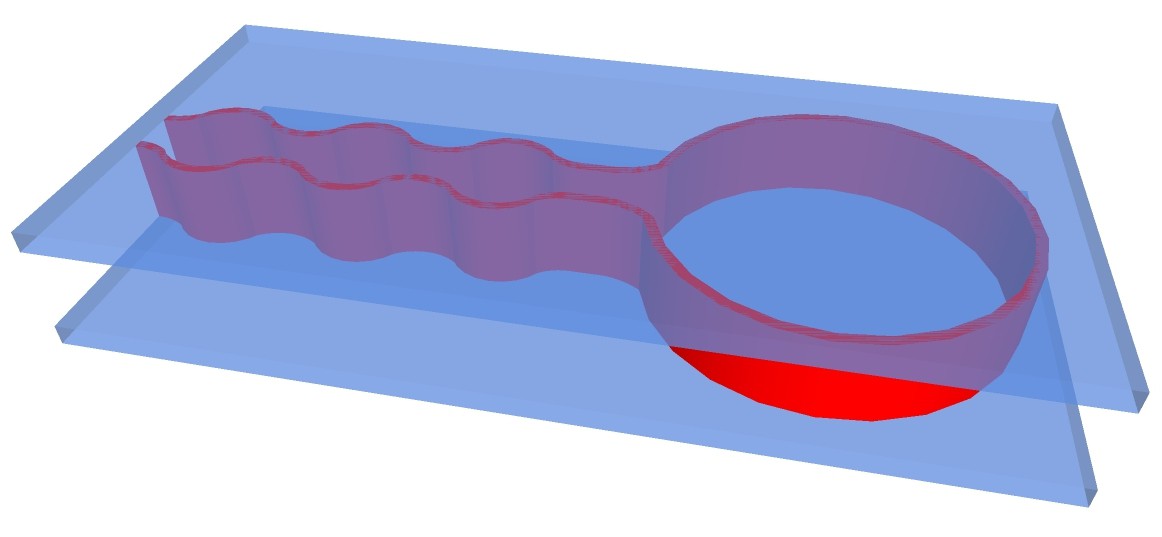
The chemputer
A chemputer is a kind of 3-D printer that 'uses a bathroom sealant as a material to print reaction chambers of precisely specified dimensions, connected with tubes of different lengths and diameters. After the bespoke miniature lab had set hard, the printer could then inject the system reactants, or "chemical inks", to create sequenced reactions'.
Well, it is basically a LoC 3-D printer, injecting automatically chemicals into it. But why not 3-D print chemical reactants itself on a near molecular level to react with eatch other on a carrier?
 M. Bindhammer
M. Bindhammer