-
Hexamine
05/26/2015 at 06:44 • 0 commentsDuring my search for cheap and easily accessible rocket fuels I stumbled over Hexamethylenetetramine, also called Hexamine. Hexamine is a white crystalline compound, which is highly soluble in water and polar organic solvents. It sublimes in a vacuum at 280 °C. Together with 1,3,5-trioxane, hexamine is a component of hexamine fuel tablets used by campers, hobbyists, the military and relief organizations for heating camping food or military rations. It burns smokeless, has a high energy density (30.0 MJ/kg), does not liquefy while burning and leaves no ashes. Hexamine has been used in colored fireworks compositions as a low-reactivity, accessory fuel.
But unfortunately I discovered that hexamine is not oxidizable by KNO₃, NaNO₃, LiNO₃, RbNO₃, Ba(NO₃)₂ and probably all other alkali or alkaline earth metal nitrates:
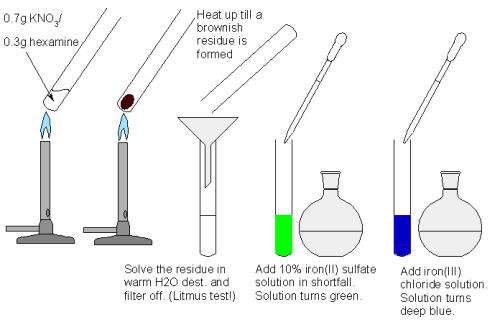
Hexamine reacts though violently with AgNO₃ but silver nitrate is not an option. A hexamine/KNO₃ mixture form cyanide's, highly basic compounds among others when enough energy in form of heat is applied, but does not burn. There are many color reactions used in cyanide determination. I have used the classical Prussian blue method:
![]()
-
The death of Dr. Wahmke
05/25/2015 at 08:59 • 0 commentsOn July 16, 1934 Dr. Kurt Wahmke and three technicians were mixing 90% H2O2 and Ethanol in a steel tank at Kummersdorf, Germany. The tank was connected to the combustion chamber of a rocket engine with a single valve between, no flame non-return finger was used. Dr. Wahmke was obsessed of the question if the use of a fuel/oxidizer mixture prior ignition would be dangerous.
The resulting explosion after ignition killed Dr. Wahmke and two of the technicians. They were the first and only deaths of technicians of German rocket development during the Second World War.
So I should be reminded how dangerous it is to mix fuel and oxidizer prior ignition in a single tank. I will use one tank for the liquefied fuel and a second one for the oxidizer.
Green eutectic rocket propellant
Environment friendly chemical rocket propellant, useable in liquid or solid state
 M. Bindhammer
M. Bindhammer