One of the major problems we face in wide adoption of electric vehicles and grid storage systems is cost. I am fixing that, and providing better batteries at the same time.
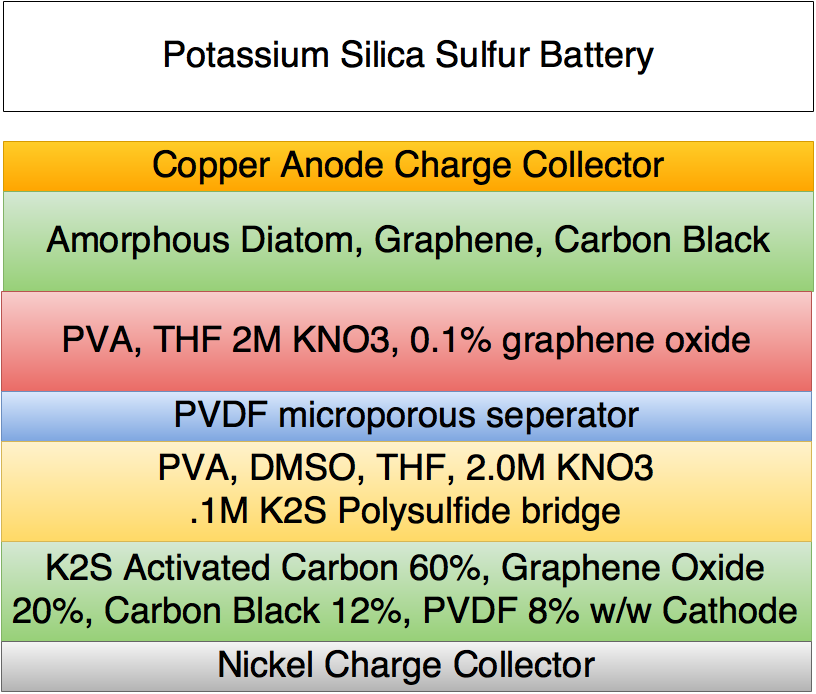
Making potassium sulfide is easy to do, all that is necessary is to heat potassium sulfate or K2SO4 with carbon and K2S and CO/CO2 are evolved. This can be completely carbon neutral in the case of using an activated carbon from coconuts.
The plan is to simply dissolve the K2SO4 in distilled water then add it to an excess of pulverized activated carbon. Dry the mixture so as to precipitate the K2SO4 in the pores of the activated carbon and then heat it in a crucible till the reaction is complete. At this time the pores of the AC will be full of K2S and can be simply combined with graphene/carbon black and assembled in a cell as the cathode.
The same exact anode as the lithium sulfur silica battery will be used. Amorphous silica is the best bet for high energy storage and food grade diatomaceous earth fits that bill perfectly. It is incredibly cheap compared to synthsized silica and has a morphology better than nano-silica being that it is highly porous and possesses a huge surface area.
A modified DMSO KOH KNO3 Diatomaceous Earth (crystalline) and Graphene Oxide electrolyte seperator will be used. This should give conductivity close to 10^-3 sieverts per cm.
This is even more advantageous as the electrolyte is much cheaper than an equivalent for lithium.
The performance will be lower than lithium sulfur silica but should still achieve over 800 mAh per gram at 2.2 volts per cell.
This documentation describes Open Hardware and is licensed under the
CERN OHL v. 1.2.
You may redistribute and modify this documentation under the terms of the
CERN OHL v.1.2. (http://ohwr.org/cernohl). This documentation is distributed
WITHOUT ANY EXPRESS OR IMPLIED WARRANTY, INCLUDING OF
MERCHANTABILITY, SATISFACTORY QUALITY AND FITNESS FOR A
PARTICULAR PURPOSE. Please see the CERN OHL v.1.2 for applicable
conditions
 MECHANICUS
MECHANICUS





 helge
helge
Hi Mechanicus.
May I ask where you got the inspiration for this cell? Several years ago I made a 'cell' accidentally and have been trying to figure out the chemistry ever since.
I've never seen crystal silicon in a battery, nowhere except semiconductors before, although I have used carbon a fair bit in my experiments.
I'm looking at your list of ingredients and thinking, most of that is probably in the flue sealant I used to create the cathode on mine.
I never made a project out of this because the materials are very hard to duplicate outside of the UK and I have a feeling its something I'll never be able to resolve due to trade secrets - I'll never know the exact chemistry. It is a shame, but thats how it goes.
Did you ever manage to get it to work? :-)
https://hackaday.io/page/3067-quantum-electron-tunneling-revisited