-
1Step 1
Make a crucible, using steel pipe. Drill a hole in one of the end caps so as to not make a pressure vessel that could come apart at the extreme temperatures a fireplace's coals can reach.
-
2Step 2
Then fill it with dryer lint and dog hair, packing it as tight as you can and saturate the packed nastiness with extra strength cleaning ammonia.
-
3Step 3
Wait for snow and cold.
Fire it in a fireplace until the pipe glows red. Leave it there for one hour.
-
4Step 4
Let the pipe crucible cool and extract the shrunken mass of reduced carbon, oxygen, nitrogen, and sulfur. The picture below is a small peice broken off of one end. The entire mass will be about 75% of the volume of the original lint/hair pack.
-
5Step 5
Mix the electrolyte. 2.0M LiSO4 and 0.1M KI. This works out to 21.998 grams LiSO4 and 1.25 grams KI in 100mL of distilled water.
-
6Step 6
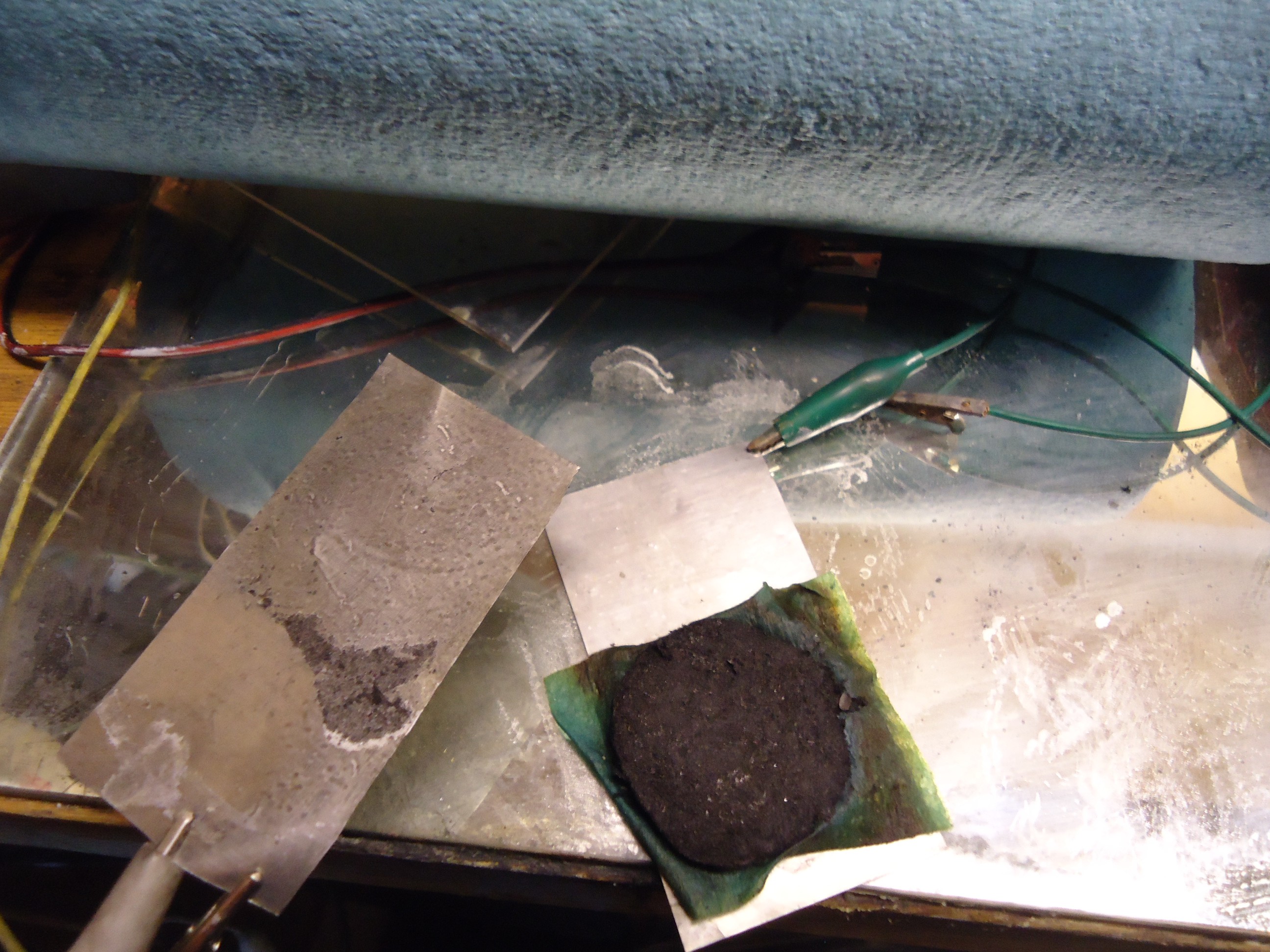
Assemble a cell by sawing a few millimeter disks off of the carbon rod. Then place a piece of shop towel between the disks.
Add electrolyte, it will readily be absorbed by the incredibly porous carbon.
And finally sandwhich between two stainless steel charge collectors.
-
7Step 7
Test!
-
8Step 8
Do the maths and graphs.
You can use my ods file to get her done. Farads are simply Coulombs divided by Volts or (Amps x Seconds)/Volts
Lint & Dog Hair Super Capacitor
A personal tale of healing, and a path towards a brighter future.
 MECHANICUS
MECHANICUS







Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.