-
Peer Review
07/11/2016 at 09:26 • 0 commentsAn updated ODT file with the correct calculations is available in the project files thanks to my contributor r.e.wolff
I am leaving this project as it stands and closing it. I will be continuing the pursuit of biomass derived supercaps in my other project.
This project was instrumental in determining my path forward and using monoliths instead of thick films for supercaps.
-
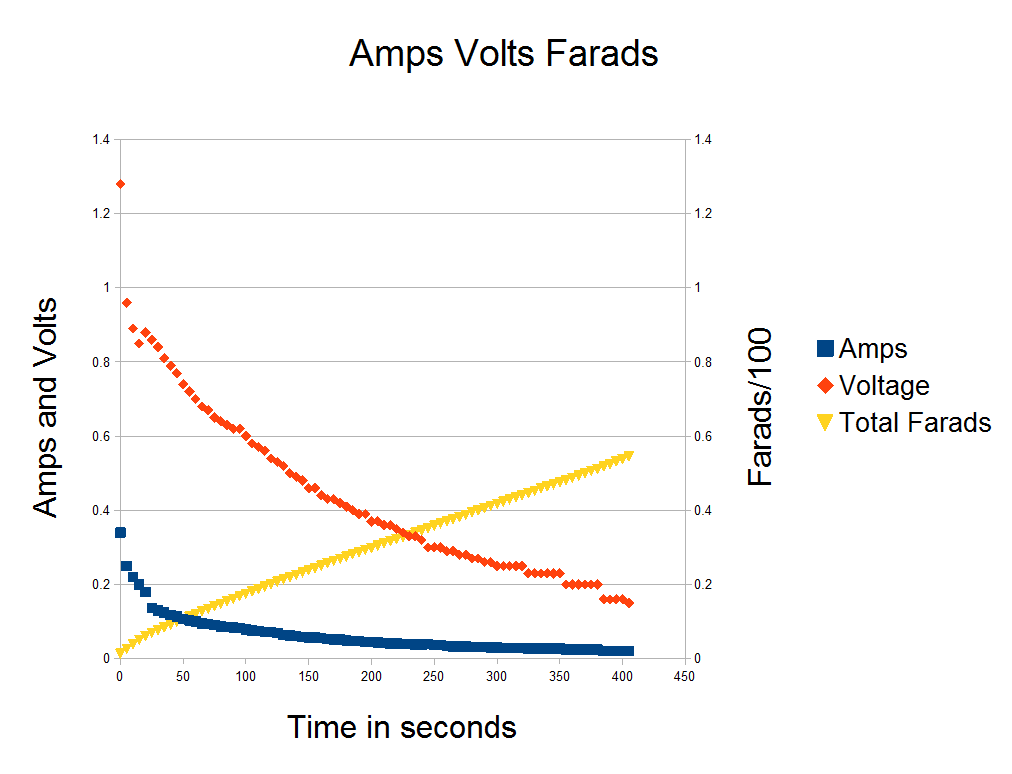
The Video Evidence
04/27/2016 at 04:57 • 0 comments -
Results are in 55 Farads per gram of active material.
04/27/2016 at 03:40 • 0 comments -
Great Success!
04/27/2016 at 00:11 • 0 comments -
Urea Addition for increased Surface Area and Carbonyl Groups
04/26/2016 at 04:45 • 0 commentshttp://pubs.rsc.org/en/content/articlelanding/2014/ra/c4ra04383a#!divAbstract
This abstract details utilizing cotton fibers and NH3 or ammonia to increase the amount of carbonyl groups that will allow more faradaic redox reactions at the cathode. The nitrogen and sulfur in hair should also be excellente' Urea can be used to generate ammonia insitu under high heat conditions. Urea comes from urine, so not only will I be using nasty hair and dryer lint I will include number 1. How is that for a gimmicky cheesy HAD prize entry?
A chinese study on hair super caps, they didn't use german short hairs though. Hail Ur-Teufel.
http://pubs.rsc.org/en/Content/ArticleLanding/2014/EE/c3ee43111h#!divAbstract
They show a massive 360 Farads per gram using the horrid electrolyte 6.0 M KOH, however it is a chinese study and in my experience they often embellish their claims by at least an order of magnitude. (cough CuCl2 ionic colloid ultracapacitor 5000 F/g cough)
Lint & Dog Hair Super Capacitor
A personal tale of healing, and a path towards a brighter future.
 MECHANICUS
MECHANICUS