-
Video!
09/24/2016 at 22:09 • 0 comments -
How does screen brightness affect DCMD response?
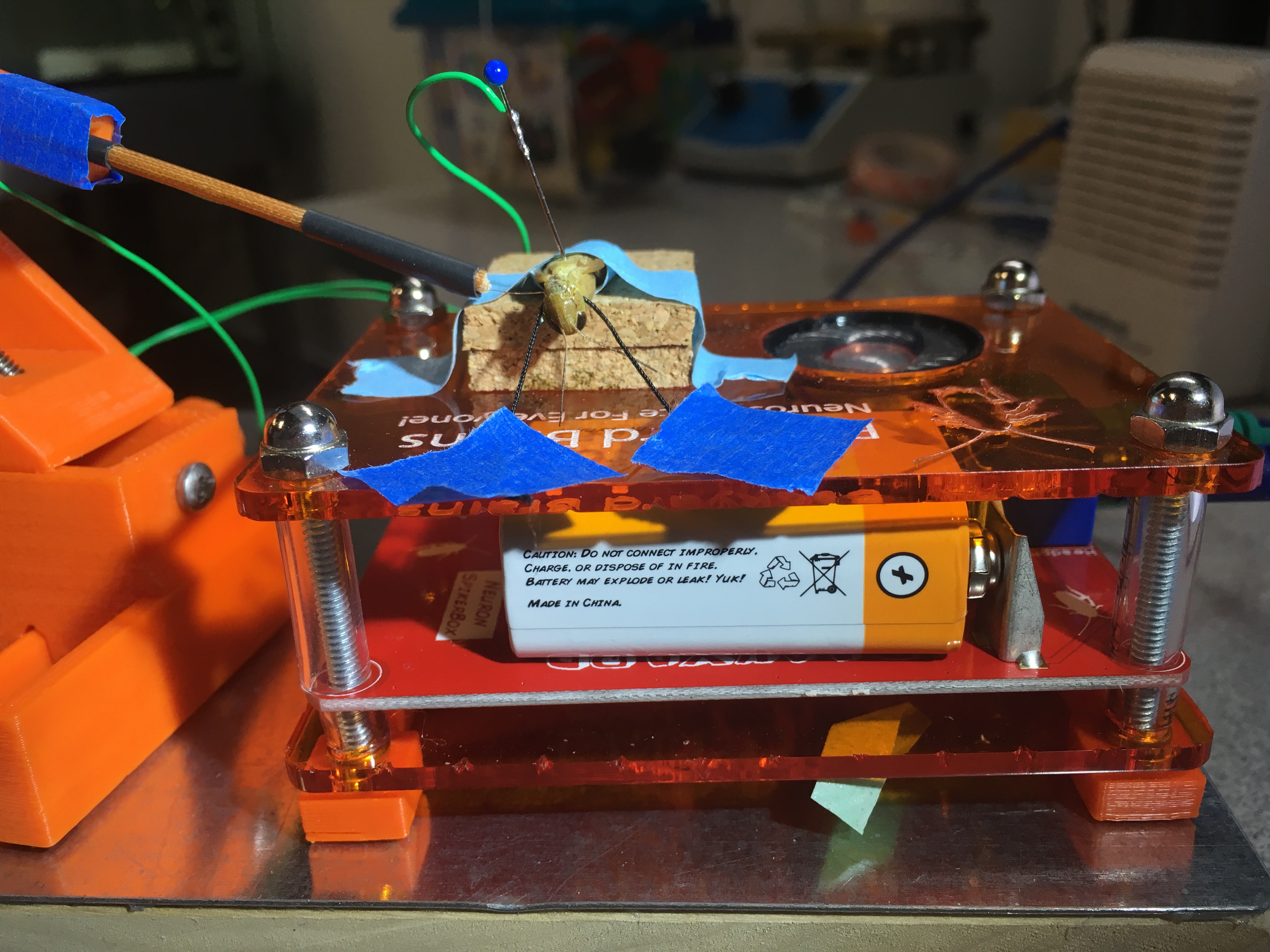
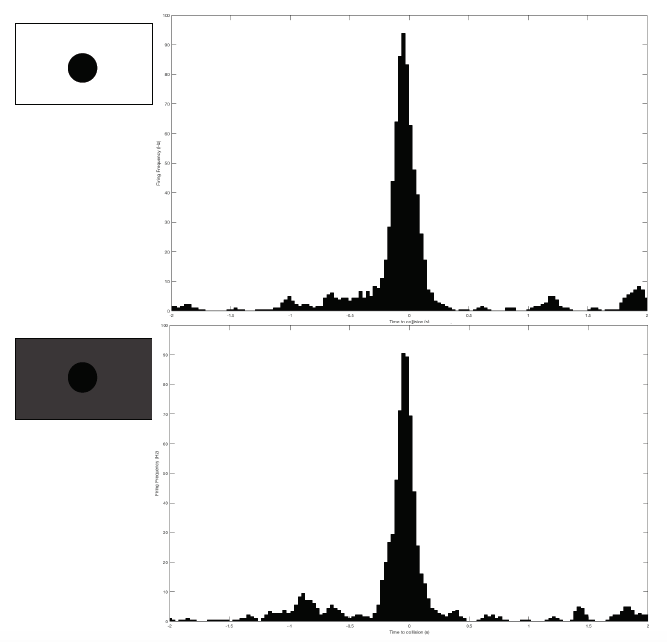
08/25/2016 at 01:30 • 0 commentsNow that I've collected ample data for the "classic" experiment of testing the DCMD response to objects approaching at various sizes and velocities, I want to keep exploring grasshopper vision. So far, the iPad screen is kept at maximum brightness, so the contrast between the white background and the black ball is high and clear. Now, can grasshoppers still see the black ball if the screen brightness is at its darkness? Let's find out!
Grasshopper G26-072516 is the subject for this test. I performed two extreme brightness levels: the highest and lowest, each for 20 trials with 6cm balls approaching at -2m/s. Note that I measured and changed the amount of brightness by adjusting that brightness bar built into the iPad. So the "lowest" brightness is not complete, pure darkness. The black ball is still identifiable, just very low contrast with the gray background.
![]()
And... I obtained results I did not expect! At max brightness, DCMD firing rate peaks at 95Hz. At minimum brightness, it peaks at 90Hz. Very, very similar firing frequency and peaking profile.
![]()
Does this mean that grasshoppers can see in the dark?! At least I can say with these negative results that grasshoppers might be able to detect approaching objects even if they din't highly contrast with the background.
-
Classic experiments: DCMD response to approaching balls
08/25/2016 at 01:09 • 0 commentsWith the ideal ITI determined, I can move on to the set of core experiments: testing to see how the DCMD neuron behaves when simulated black balls of different sizes and velocities approach the grasshopper's exposed eye. So my little friends spend about 2 hours on top of the SpikerBox for these experiments.
![]()
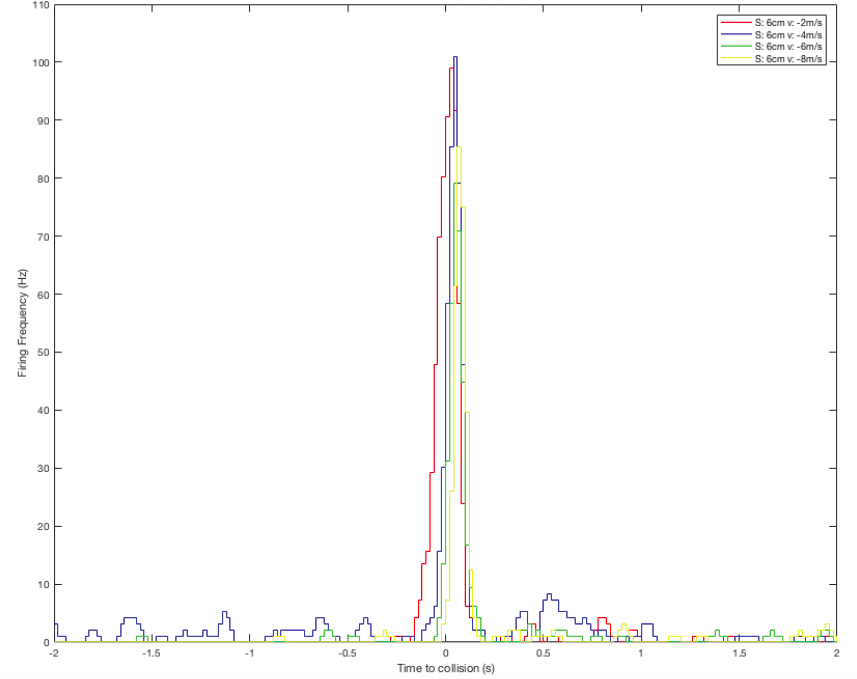
I continue to process the data in MatLab for better visualization. Here are the results for balls approaching from a constant initial distant of 10cm, 6cm in size, and with various velocities (-2, -4, -6, -8m/s).
Perievent histogram: showing DCMD firing frequency 2s before and 2s after the simulated collision between the eye and the object:
![]()
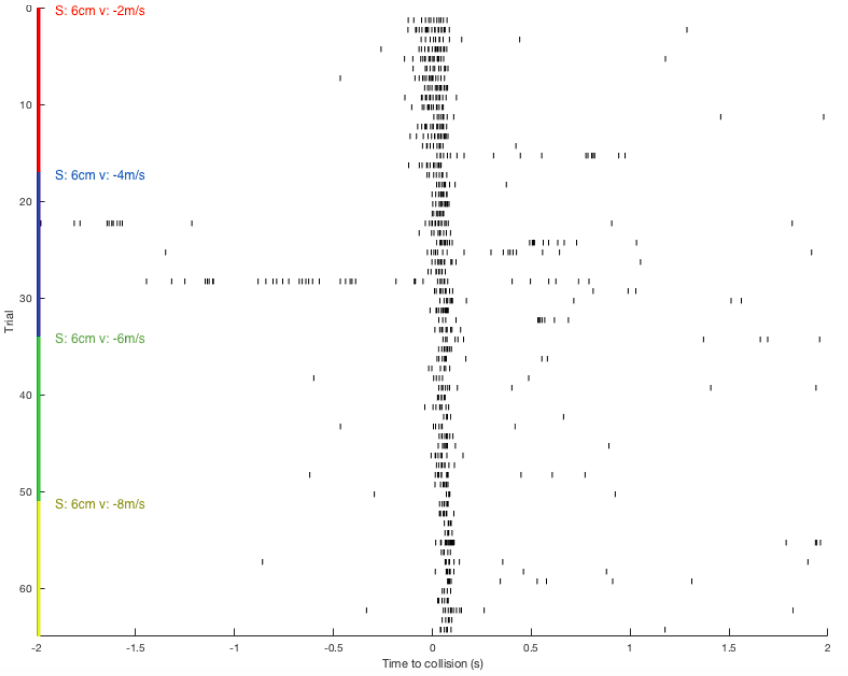
Raster plot: showing DCMD spiking pattern across each pair of S and v over time. DCMD firing peaks around collision for objects approaching at -2m/s, and after collision for objects approaching faster:
![]()
-
New & improved ITI test
08/25/2016 at 00:53 • 0 commentsIn the 'Preliminary data' log, I had begun my data collection and analysis journey. I first performed the intertrial interval, or ITI, test, to determine the ideal time between 2 stimuli so that the time is long enough to avoid the grasshoppers' habituation to the simulated balls. The results figures I showed in that previous log showed that the 45s ITI was better than the other ITIs in giving us a nice profile of the DCMD neuron activity over time. However, of course, the data visualization could be much improved, and I have been doing that by importing the recordings (stored in JSON files by the SpikeRecorder app) into MatLab (using JSONlab). MatLab yields cleaner and to-scale figures that give us an even better idea of the DCMD profiles in different ITIs.
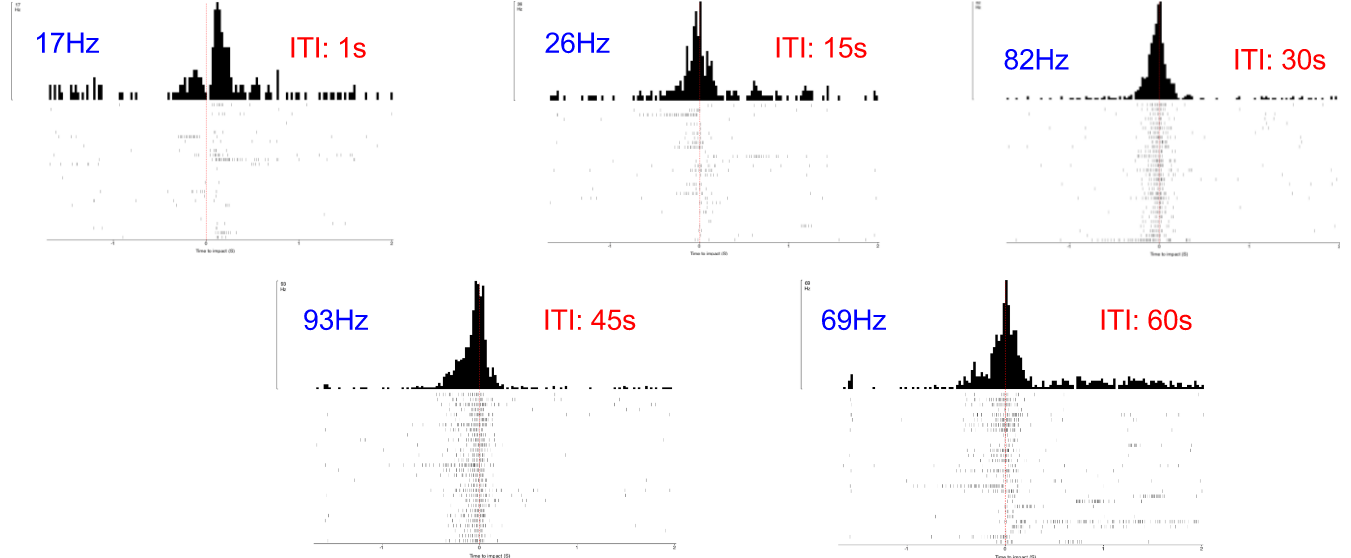
Here, compare! These are the old figures, not to scale and all are the same height. So I had to label them all with their frequencies:
![]()
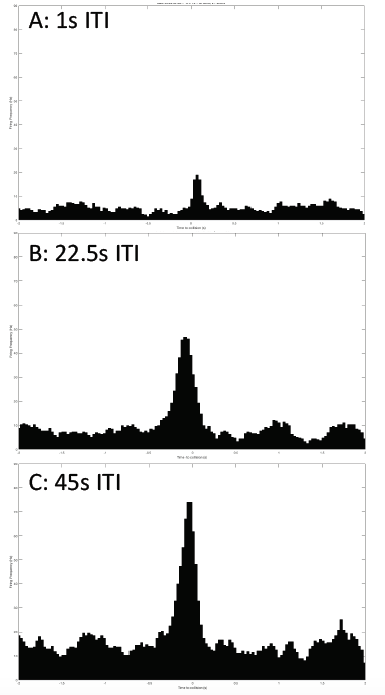
I performed a new ITI experiment on a new grasshopper, G25-072416-01. This time, I used 3 different ITIs that I think are sufficient: 45s, 22.5s, and 1s. All other experimental parameters are kept constant: iPad screen is 0.10m from the grasshopper's eye, balls of 0.06m radius approach at -2m/s (negative for the increasingly shortened distance between the eye and the object). 30 trials per ITI test. And the data is processed in MatLab, and it looks beautiful!
Sorry the axis labels are too small to read. Horizontal axis: time to collision, from -2 to 2 seconds. Vertical axis: Firing frequency in Hz. Firing frequency is much higher in the 45s ITI, making it a "good" ITI to use for the subsequent experiments.![]()
-
Recording live neurons: the SpikeRecorder app
08/24/2016 at 23:31 • 0 commentsIn the project instructions, I've briefly talked about the BYB SpikeRecorder app that I've been using on an iPad to add to my grasshopper vision project the flavor of a low-cost-and-DIY-albeit-of-great-quality tool. Here, I'll talk about it in a bit more details to give the spotlight to one of the main components of my project.
Firstly, the purpose of the original SpikeRecorder version that BYB has published is to record data directly to your PC (or tablets & smartphones) while you can observe the recording in real time. There's also the functionality of saving the recording to be played back anytime. And if you're familiar with the classic model of an action potential (aka spikes!), the SpikeRecorder also allows a threshold view, where you can set your threshold and get a snapshot of your spikes.
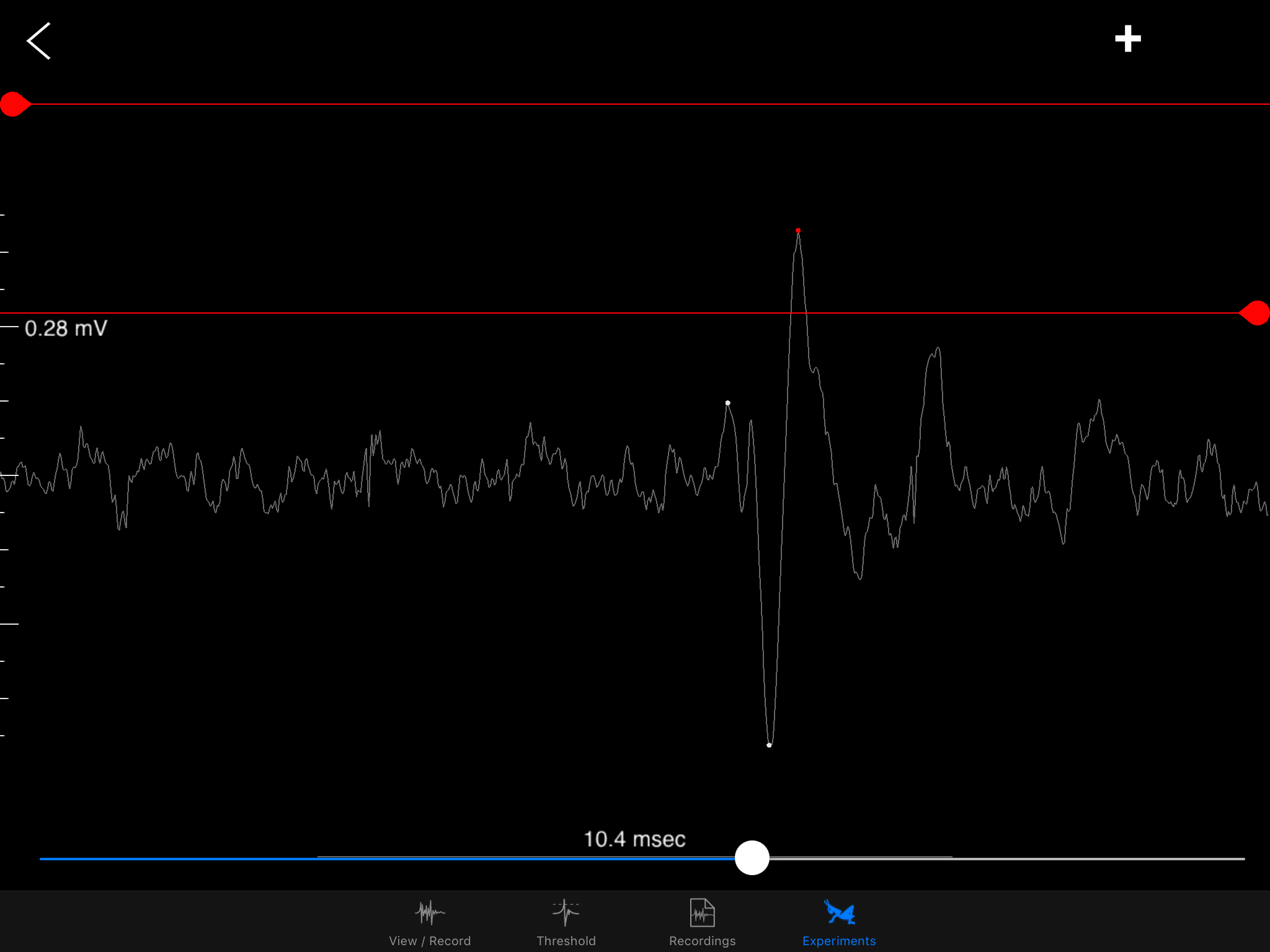
This is a classic "spike" event when the electrochemical properties of a neuron is at work. These spikes are essentially changes in voltage due to the chemical and electrical difference inside and outside of a neuron's membrane. Movements of sodium and potassium across the membrane via channels and the way their charges get distributed -- these are the main components of a spike.
![]()
Art by Backyard Brains
If you're interested in checking out this app and perhaps get some spikes, the app is available for android and ios. And of course, the code is on github for the open source spirit!
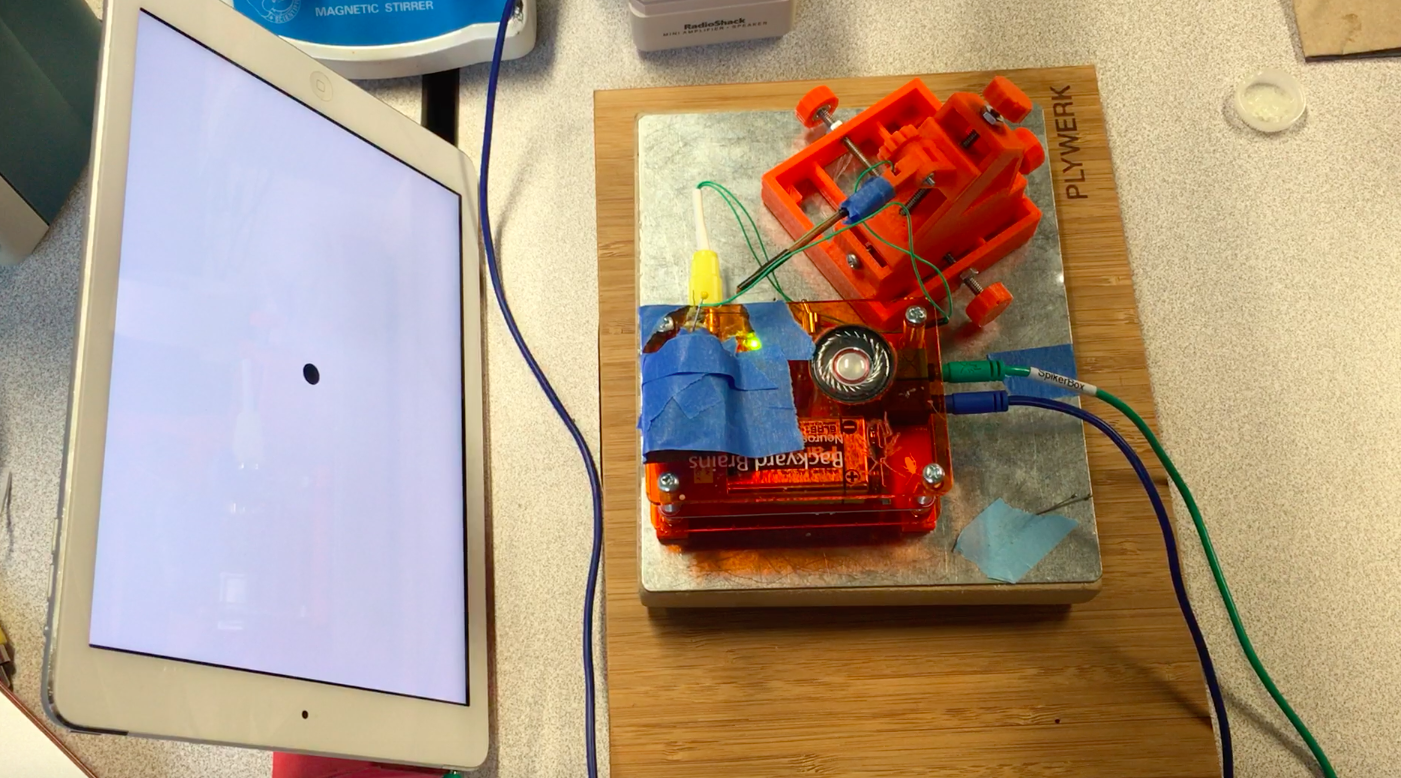
One of my mentors, Stanislav Mircic, is the computer science god of BYB. He graciously added the "Grasshopper experiment" functionality to the app. The app now can provide both the visual stimuli (simulated balls thrown at grasshopper's eye) and recording/analysis of the DCMD neuron activity.
![]() Sorting a bunch of spikes at once:
Sorting a bunch of spikes at once:Zooming into one DCMD spike!
-
A new naming system for database!
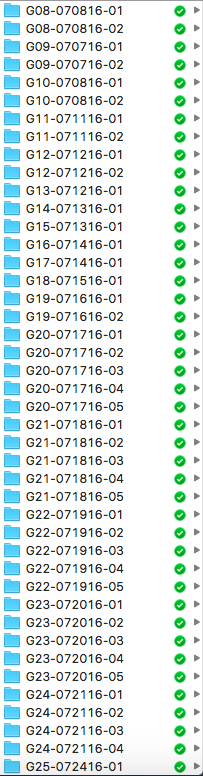
08/24/2016 at 23:12 • 0 commentsAs I experiment on more and more little grasshoppers, I realize the importance of organization skills. Specifically, I'm talking about how messy my housekeeping of the recordings and analyses have been. In an earlier post, I wrote that my naming system for each grasshopper is in the following format: [day][month][letter indicating order in the day]. While a name of 2408A isn't terrible, what my mentor Greg Gage came up with in a minute is significantly better. (And sitting down with him to discuss my preliminary results also jumpstarted the task of organizing folders and files and sharing in Dropbox.)
So, now each grasshopper has the following name format: G[number]-[month][day][year]-[test number]. So, G08-070816-01 denotes that the folder containing recordings belonging to the 8th grasshopper I've tested on, on the 8th of July in 2016, for the first test. A second or third test could follow, and new folders are made to keep the data for those tests. So my database is now much more organized:
![]()
While this log is not about building or experimenting or data, it's about a skill that anyone, especially scientists, should have. I can imagine all sorts of problems if all my recorded m4a files stayed in the chaos from before: wrong data analyzed, data from different grasshoppers get mixed up, etc. Good thing I sorted this out before entering the point of no return.
-
Preliminary data
07/11/2016 at 02:56 • 0 commentsAfter trials and errors, the electrophysiology setup is ready to collect usable data. I have updated the current version of the protocol and setup on the instructions tab.
For housekeeping, I give each grasshopper that participates in the experiments a name in the format of [day][month][letter indicating order]. For example, a grasshopper whose DCMDs are recorded today would be named 0710A. If I request the help of another grasshopper, that subject would be 0710B.
The first series of experiments I am performing aims to record and analyze the activity of the DCMD neuron when the black ball is approaching the grasshopper's eye. My hypothesis is that the neuron's peak firing/activity rate would be around the time when the simulated ball would hypothetically collide with the eye.
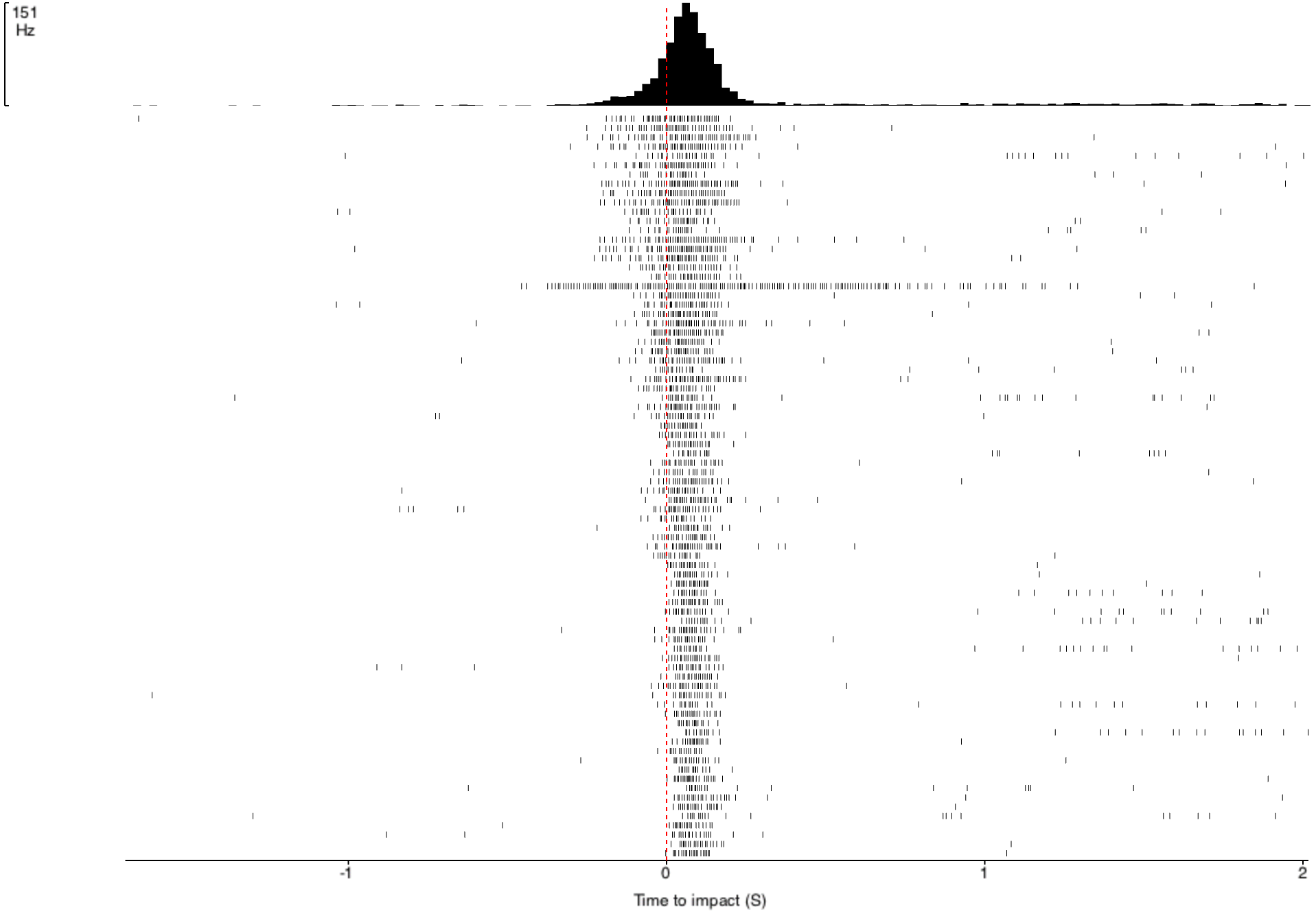
Before I performed the first of these experiments, I needed to determine the "ideal" intertrial interval, ITI, when no visual stimulus is present. This interval between two stimuli is necessary due to the possibility of habituation to the stimuli. If the ITI is too short (e.g. 1 second), the DCMD neuron might no longer consistently fire. I need to quantify the neuronal responses to different intervals and identify an ITI that will be long enough for the neuron to respond to most or all of the approaching balls. The ITIs I chose are: 1, 15, 30, 45, and 60 seconds. All other experimental parameters are kept constant across all ITI tests: with the iPad screen 0.10m from the grasshopper's eye, balls of 0.06m radius approach at -2m/s (negative for the increasingly shortened distance between the eye and the object). 30 trials per ITI test. Here are the results!
![]()
- Horizontal axis: 0 (red dash line) is the time of impact/collision between the eye and the ball. The graphs show the DCMD activity 2 seconds before and after the impact.
- Vertical axis: Two representations of the clusters of the DCMD spikes, which mostly are around the time of impact. Other "spikes" more than 0.5sec from 0 either direction are most likely noise, a common trouble for electrophysiology recordings, or other strange neuronal activity.
As you can see, the DCMD firing rates for the ITI of 1sec and 15sec are low and less consistent compared to the rest of the ITIs. The 45sec ITI yields relatively the best profile of the DCMD activity, and so I will use this ITI for my first series of experiments.
In this series of experiments, these are the constants across all trials:
- Distance from the grasshopper's eye to the iPad screen: 0.10m
- Intertrial interval: 45sec
- Object size: 0.06m
- Trials each pair of object size and velocity: 16
- Total experiment time: 60min
- The approach velocities are varied: -2, -4, -6, -8, -10m/s. Each ball is a combination of the object size and one of the velocities.
Preliminary results:
As expected, DMCD peak activity clusters around the time of impact, at 0sec. Interestingly, this peak is about 90msec after the supposed time of impact. Some questions I must ask are: Does this result make intuitive sense, when the neuron supposedly acts as a warning and escape mechanism and its activity should hypothetically peak before the collision so the animal would jump away to avoid being hit? Is the iPad screen big enough and close enough for the grasshopper to really "sense" the danger of collision? What are the possible factors in the iPad app that might yield this result? Is the simulated time of collision (when the ball of a particular size stops expanding) accurately computed and depicted on this graph? I will continue to investigate this and make appropriate adjustments.
-
Experimental Setup & Data Collection Begins!
07/06/2016 at 01:39 • 0 commentsMaterials: check. Grasshoppers: Check. Protocol: Check, and please do check the instructions on the main project profile for the protocol of this experiment. Next step: Setting up the experiment and take off!
This is how the grasshopper spends an hour of its time for science:
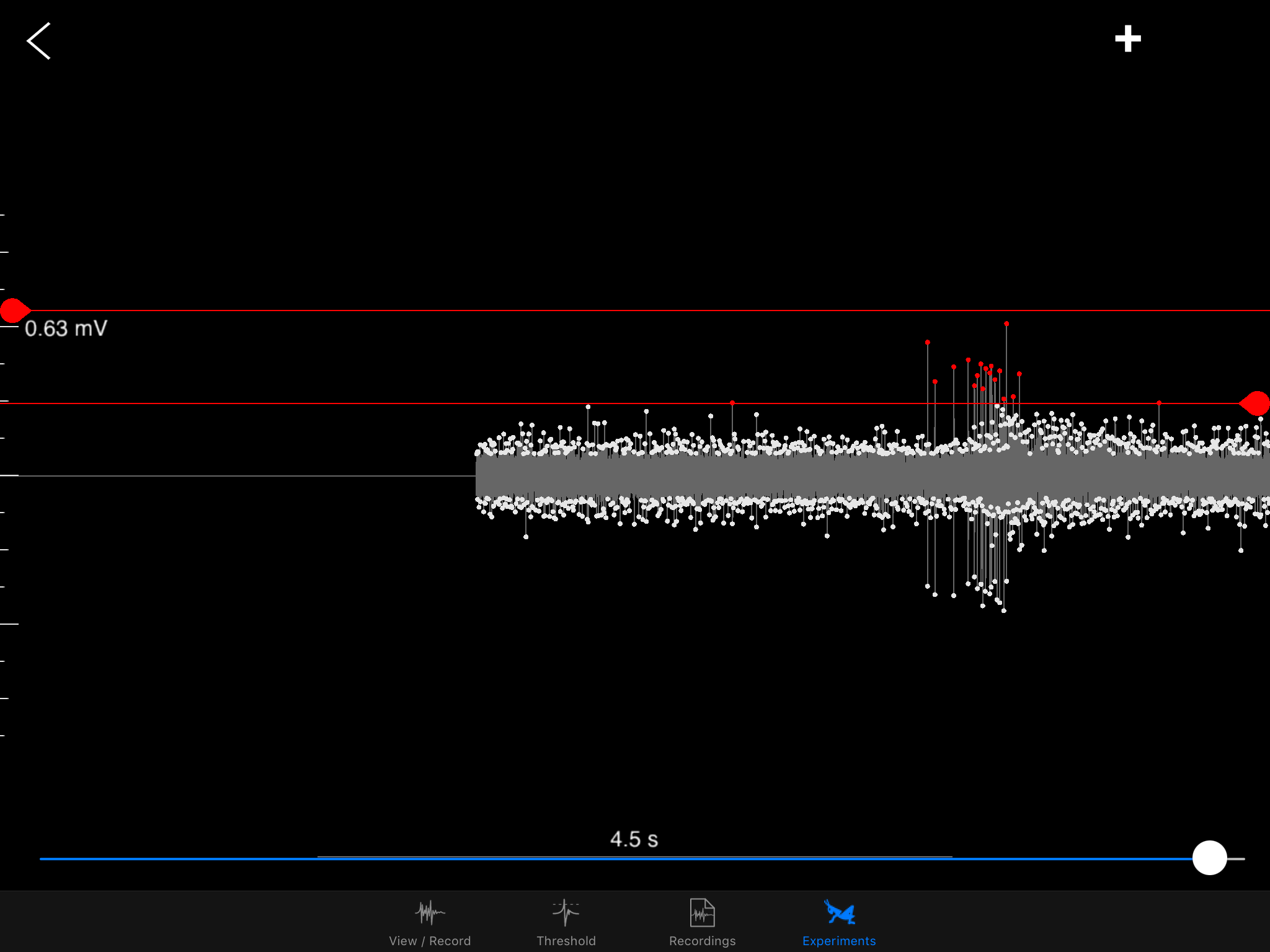
The iPad screen is placed on the side contralateral (opposite) to where I place the electrode on the grasshopper's neck. Here, the electrode is on the grasshopper's right side, so the iPad is on the left. In the blurry background, you might see a white RadioShack mini speaker, which amplifies the signal sounds and helps me identify neural spikes the DCMD neurons generate during the experiment. Electrophysiology is half seeing the spikes on the oscilloscope (or in this case, the iPad app that can do it all) and hearing them. And the spikes are distinctive! Here, see and listen to my initial test of the setup. In this test, the ball's radius is 6cm (0.06m) approaching at 3m/s.
Do you hear it? As the black ball gets closer and closer, at a certain size of the ball, there's a swoosh or krrrrr (or whatever you heard) sound that stands out from the base noise of the recordings. That's the DMCD spiking! In the screenshot of the recordings above, the spikes standing tall and distinctive are marked by the red dots. In the field, the grasshopper would probably jump away when the neurons fire, to avoid colliding with whatever object that's coming toward it. In my Backyard Brains lab, by cooperating and responding to the simulated ball, the grasshopper is greatly contributing to vision neuroscience at large and my experience with insect electrophysiology and neuroscience in particular. So, thank you, little grasshopper.
After I tested the setup and heard those exciting initial spikes, I added a few more finishing details to the setup. As of now, the grasshopper will get a room of its own whenever the experiment is conducted. The lights will be off, so there is sufficient intensity of contrast of the black ball against a white background of the iPad. (Perhaps as followup questions and studies, I can test whether grasshoppers can identify approaching objects in a cluttered background, or what colors can these bugs see.) Noise from other electronic equipment and devices will need to be minimized and thus those devices will be turned off, for optimal signal:noise ratio.
Thus, the experiment is conducted in darkness like this:
I will collect data from now on. Stay tuned for an update on the data!
-
Catching grasshoppers!
06/29/2016 at 02:44 • 0 commentsI went out to the field in Ann Arbor, MI yesterday and in my mind, I wanted to catch at least 20 grasshoppers to last me about two weeks of data collection. After two hours of navigating through a vast tall grass field in the Nichols Arboretum in the scorching summer heat, I had to lower my expectation, to maybe about 1 little grasshopper in my cage and I'd happily go home.
The grasshopper's abilities to camouflage and escape are responsible for my wasted time. But I shouldn't think of it as wasted time! After all, I am studying one of the reasons why these bugs can escape dangerous predators, like myself who will make the grasshoppers watch circles expanding and contracting on the iPad screen.
Today, with practice and learning from the WikiHow article on how to catch grasshoppers, I became a capable predator. With one hand, I now can simply come from behind a grasshopper sitting on a leaf and enclose my hand with both leaf and grasshopper wiggling inside. They don't only struggle to escape from my hand, they also vomit a tobacco-colored bile as part of their escape mechanism after being caught. (So many escape methods! I study the mechanism they use to avoid being caught in the first place.) A paper studied this regurgitation behavior as the self-defense of the grasshoppers and found that the vomit can make lizards reject the grasshopper before complete ingestion. But the vomit might not protect the insect from vile humans, at least not when the human is me, determined to catch my study organisms despite the discolorations of my hands.
![]()
For a successful catch in a tall grass field, sweep through the field despite the itch and the thorns. Use both the central and peripheral visual fields. Spot the grasshoppers who blend in too well with the plants and ground (they come in many shapes and colors--mostly green and brown). And be decisive! Grab and pluck that leaf that the alien-eyed bug is sitting on. And ta-da! Happiness.
![]()
-
Designing Experimental Setup & Gathering Materials
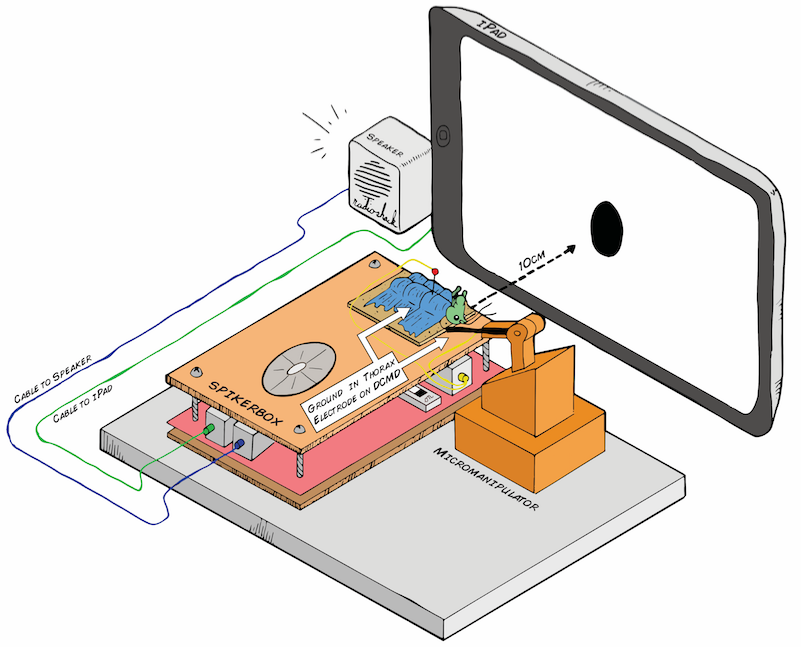
06/25/2016 at 18:50 • 0 commentsBelow is the preliminary design for my electrophysiology setup. The Backyard Brains SpikerBox has a piece of cardboard on top, so after being anesthetized in ice, the little grasshopper will chill out there for the experiment, which would last about an hour. The grasshopper's belly (ventral side) would face up, so I can place the recording electrode in its neck (where the activity of the DCMD neurons can be picked up) and the reference in either its abdomen or thorax, where I assume the DCMD activity would not be found. Because the DCMDs are theoretically responsive to "contralateral" stimuli that is on the opposite eye, if I place the electrode (using the Backyard Brains 3D printed micromanipulator for precision) on the right side of the grasshopper's neck, I would expose its left eye to the iPad. I will use a level ruler to make sure the angle between the center of the screen (also the center of the stimulus) and the center of the grasshopper's eye is as minimal as possible.
![]()
Art by Tanner @ All Hands Active, MI
During the trials, the iPad screen will throw a black ball (in a white background for stark contrast) at the grasshopper's eye. Some parameters I can control are: distance between the ball/screen and the eye, the size of the ball, and the ball's velocity of approach (ball expanding in size). What will happen when a small ball is approaching the eye slowly? When it is fast? When the ball is big? We'll see!
The profile page of this project includes the full list of the materials I need and the detailed instructions, from preparing the little grasshoppers for the experiments to throwing simulated balls at them to recording their DCMD activity.
Neuroscience of Grasshopper Jumps
Why are grasshoppers so hard to catch? Let's explore the visual neurons behind the grasshopper's escape mechanism!
 Dieu My Nguyen
Dieu My Nguyen

 Sorting a bunch of spikes at once:
Sorting a bunch of spikes at once: