-
1Remove copper coating from back of pennies
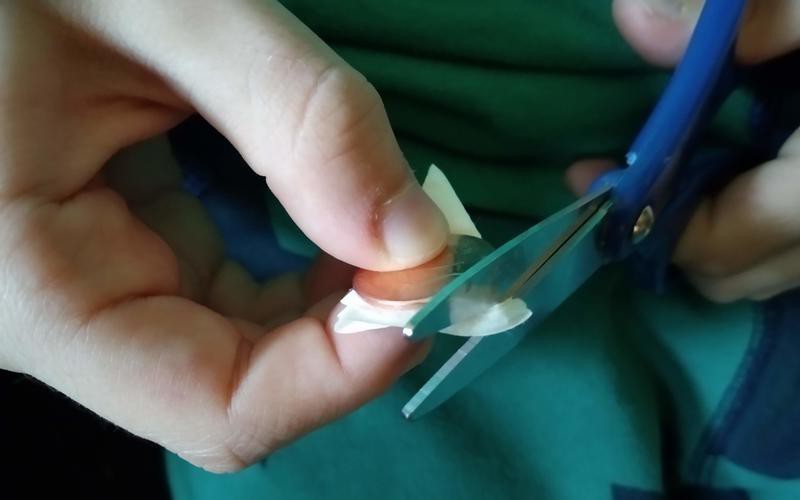
Use regular sandpaper to remove the thin copper coating from three or four US pennies, minted after 1982. Newer pennies are made from pure zinc with a thin copper coating.
![]()
![]()
There's about seven or eight minutes of sanding between the photo above and below.
![]()
It took us about half an hour per penny. After three pennies it was getting dark, and we were out of patience, and expendable thumb nails.
![]()
We heard somewhere that it's even harder to remove the copper on the front (heads) than the back (tails). Tails was hard enough.
![]()
We tried both coarse and fine grit, but didn't see much difference.
There must be a better way!
-
2Make a separator for each cell
We used AeroPress coffee filters for separators.
![]()
Folded in half three times they become about the size of a penny.
![]()
Trim off the excess paper.
![]()
![]()
-
3Soak the separators in household vinegar
We used pure household vinegar (5% acetic acid) as electrolyte. It is common to add some table salt (sodium chloride) to improve conductivity, and perhaps to mitigate partial passivation of the zinc anode. To keep the chemistry as simple as possible, we went with pure vinegar.
![]()
-
4Attach leads to pile ends and assemble the pile
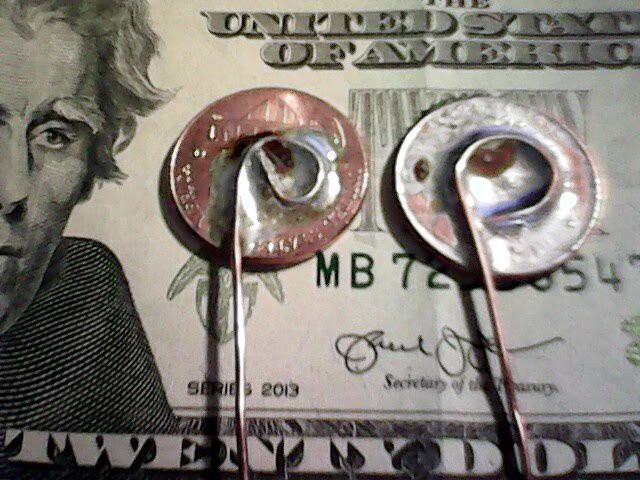
We soldered copper wire to both pile-end pennies.
![]()
![]()
Note that one end of the pile (the positive one) has a penny with fully intact copper coating.
Now put the whole Volta pile together!
![]()
Four pennies (one intact, three with zinc tails), three separators: a three-cell Volta pile.
The clothes pin was pinching the pile a bit too hard and squeezed out the electrolyte.
-
5Use the Volta pile to power a red LED
Measure the voltage.
![]()
Almost 1.7 V, or 0.55 V per cell. Theoretically you could get as much as 0.76 V per cell.
Is it enough to light up an LED?
![]()
Yes! It looks like our Volta pile is able to pull about five mA through a red LED. With table salt added to the electrolyte, you could probably make it brighter.
Science-Fair Penny Volta Pile
My son and I did this 3-cell, 4-penny Volta pile for his 3rd grade Science Fair. His school is closed, so we're putting the project online.









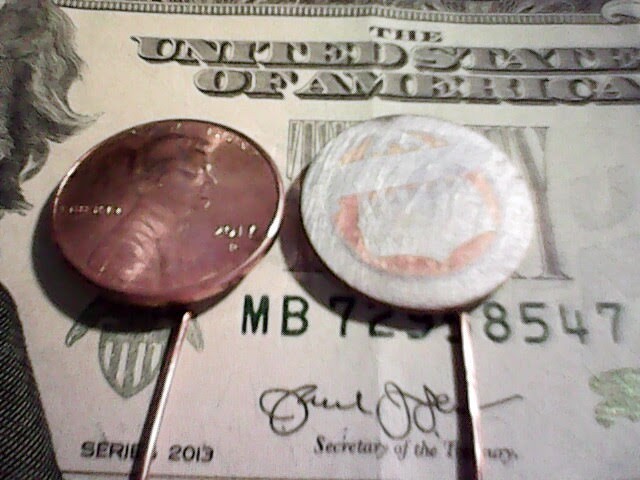



Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.