21/07/2023, Friday, 10:16.
For some reason this last week got me too lazy to do anything at all...
Well, I will just leave this here and see what I can think of...
----------------------------------------------------------------------------------------------------------
Well, I wrote the DIY iron-air battery with a "¹", because I thought I would be able to understand and replicate the papers that I saw...
But, as you can imagine, I did read stuff, and it all came to: either I don't have the equipment to replicate their methods, either they didn't talk about their methods.
Soooooooo... It is what it is, I suppose.
----------------------------------------------------------------------------------------------------------
Well, even though I'm trying to make the "sketch" of the mech with these project logs since Project Log 54, I don't quite know where to go from here.
https://hackaday.io/project/188391-diy-mechexoskeleton-suit/log/220205-project-log-54-just-one-last-try
I think I will make this project log about the linear actuators of the mech/exosuit.
Like in project log 58, were I talked about zip chain actuators for both flexible and rigid linear actuators, I believe I will stick to that idea.
https://hackaday.io/project/188391-diy-mechexoskeleton-suit/log/220958-project-log-58-diy-electric-motor
One thing I never liked about the hoist/winch mechanism is it lack of efficiency, which the zip chain would (supposedly) solve.
But now I think I got myself in another little conundrum.
You see, since the zip chain actuator is, well, a chain actuator, the logics of torque, speed and so on will still be applied to it.
And thus, I can reach a point that I don't really need the reduction gear from James Bruton which I was thinking on using.
Which needs to be calculated, and right it is Sunday, and I'm not in the mood, lol.
----------------------------------------------------------------------------------------------------------
PS: I talked about the rack and pinion to the ChatGPT because it is easier to calculate and it is basically the same thing.

I asked ChatGPT:
"ChatGPT
To determine the number of teeth required for the rack and pinion mechanism, we need to consider the relationship between rotational motion and linear motion.
For a gear, the linear speed (V) can be calculated using the formula:
V = π * d * RPM / 60
Where:
V is the linear speed
d is the diameter of the gear
RPM is the revolutions per minute
In your case, with a gear diameter of 10 cm and an RPM of 30, the linear speed would be:
V = π * 10 * 30 / 60 = 15π cm/s ≈ 47.1 cm/s
Now, if you want a 1:1 output, the linear speed of the rack (which is the same as the pinion's linear speed) should be equal to 47.1 cm/s. Therefore, you need to choose the number of teeth on the rack and pinion such that the linear speed matches.
Let's assume the number of teeth on the pinion is P and the number of teeth on the rack is R. The gear ratio (GR) can be calculated as:
GR = R / P
For a 1:1 output, the gear ratio should be 1. Therefore:
1 = R / P
Now, to achieve a 10:1 output, the gear ratio would be 10:
10 = R / P
To find the number of teeth, we need to consider the pitch radius (radius corresponding to one tooth) of the gear. The pitch radius (Rp) can be calculated as:
Rp = d / 2
Where:
Rp is the pitch radius
d is the diameter of the gear
In your case, the pitch radius would be:
Rp = 10 cm / 2 = 5 cm
Now, we can calculate the number of teeth using the following formulas:
For 1:1 output:
P = R
For 10:1 output:
P = R / 10
The number of teeth can be calculated using the formula:
T = 2 * π * Rp / pitch
Where:
T is the number of teeth
Rp is the pitch radius
pitch is the pitch of the teeth (distance between adjacent teeth)
The pitch can be calculated as:
pitch = linear speed / RPM
Let's calculate the number of teeth for both scenarios:
For 1:1 output:
pitch = 47.1 cm/s / 30 RPM = 1.57 cm/tooth
P = R = 2 * π * 5 cm / 1.57 cm/tooth ≈ 6.37 teeth
Since the number of teeth cannot be fractional, you might need to round up or down to the nearest whole number. In this case, you could use 6 teeth for both the pinion and rack.
For 10:1 output:
pitch = 47.1 cm/s / 30 RPM = 1.57 cm/tooth
P = R / 10 = 2 * π * 5 cm / 1.57 cm/tooth ≈ 0.637 teeth
Again, rounding up or down to the nearest whole number, you could use 1 tooth for the pinion and 10 teeth for the rack.
Please note that these calculations assume an idealized scenario, and in practice, you might need to consider factors such as backlash, tooth strength, and smoothness of operation in your gear and rack design."
----------------------------------------------------------------------------------------------------------
Well... I didn't understood a hint on what the heck ChatGPT was talking, but I think I kinda got the gist of it.
Basically, if I were to make a 10:1 ratio, I would need to take into consideration both the diameter and number of teeth.
If the gear/pinion has a number of teeth "X" (let's say, 10 teeth), the pinion and the rack should have the dimensions (size, length etc) allowing for the rack move a "Y" amount of length and the pinion rotate a "Z" amount of times to allow everything to rotate the distance you want.
A giant 2 meter wide gear/pinion with 10 teeth would move way faster at a rack with 20 teeth and 1 meter of length (assuming the physics would work out for this hyphotetical example) rather than a gear with 10cm of diameter and 20 teeth in a rack with 100 teeth and merely 30cm of length.
I guess one easy way of making a rack and pinion would be to use the circunference of a circle multiplied by the times you want the reduction to occur and then add the same number of teeth that would allow for the reduction.
So, a 10:1 reduction ratio would force me to take a 2cm diameter pinion, which would be 6.28319cm, then insert 5 teeth on the gear and other 50 teeth on the rack with 62.8319cm of length. (I think).
... Or not, bruh, this is wrecking my brain.
----------------------------------------------------------------------------------------------------------
I did found a promising open source zip chain actuator, the problem is that I'm starting to doubt whenever or not this aproach is really practical.
https://www.thingiverse.com/thing:1283288
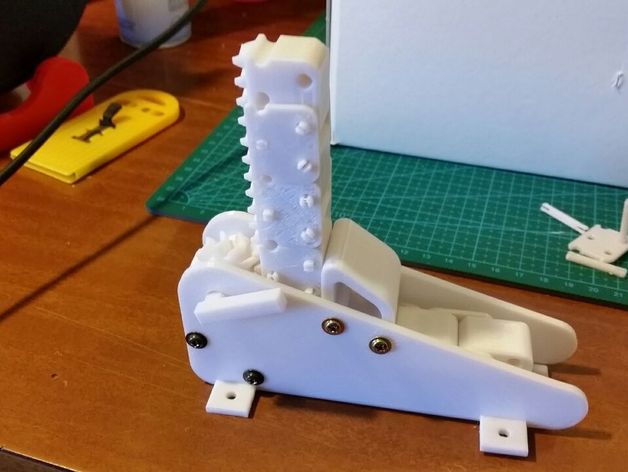
I think I will be forced to keep up with the old hoist/winch mechanism... But I don't trust on its efficiency... y=y
By the way, the article chooses the C option. But I would keep with the B option, a compound pulley loses 5% to 15% of efficiency per pulley, and the third kinda is a compound pulley.
The problem with telescopic cable-driven actuator is not lifting, it is pulling. You need a double actuation for this to be useful, and how do you do that?
The article just showed: put a hoist connected to the tip and another connected to the pulley system.
----------------------------------------------------------------------------------------------------------
Also, I just saw this eccentric gear and I quite liked it.
Although you can't really use planar laser cutting for it, I do like its simplicity.
It is a 1 teeth gear with a 10 teeth bigger gear, giving it a 10:1 gear reduction ratio in an apparently studier and simpler gear than a cycloidal gearbox or a conventional spur gearbox.
This guy teaches how to program/calculate an eccentrical cycloidal gear.
However, I would advise you to make a version that is way thicker and with a double helical shape.
----------------------------------------------------------------------------------------------------------
By the way, one important thing: all these actuators will be using bearings and all of these bearings need to sustain the entire weight of the suit.
I was thinking on using car bearings, but these are kinda expensive to simply go buying around, but I have an idea.
Basically, cross roller bearings are a well-used type of bearing that is normally meant for heavy duty applications, even the Hacksmith used those on the hands/arms of their 6 ton lifting power loader.
I will be using this guy's tutorial.
Of course, you can just buy ball bearings on the internet, these are kinda cheap and come in the hundreds.
I'm leaving this here to make use of it later, for some reason I feel way more indisposed than usual right now...
----------------------------------------------------------------------------------------------------------
Bruuuuh... I'm really not on the mood right now to 3D model the actuator... But I think I will stay with the linear hoist actuator... Or not... Every time I think about it I feel like I'm overlooking a lot of stuff, every time I think I should switch to hydraulics or the reversebly actuated pneumatic artificial muscle instead...
I say this because the linear actuator will be under compression, but an hydraulic actuator will transfer the loads to the HDPE composite as tension on its walls, which is the strength of the HDPE.
Actually, no...
I just researched the overall characteristics of HDPE and UHMWPE (or HMPE) and it showed extremely different characteristics from which I remember seeing/posted here before.
Unfortunately, I didn't straight up wrote the values, but it were close to 90 MPA, now I can only find links saying that HDPE can only hold up to 26 MPA of tensile strength and up to 30 MPA of compressive strength.
------------------------------------------------------------------------------------------------------------------------------
Well, while I got disappointed with my own mistake of simply ignoring the problems until they hit my face, I got wondering how I could surpass said problems.
If you're not catching my drift, it is the fact that the HDPE and/or UHMWPE plastics, although very useful, are still plastics. So they may or may not be capable of being used as the main structure of the Mech/Exosuit.
If you convert the compressive/tensile strength that these have of +/- 30 MPa to bar or KgfCm², you would only get a maximum strength of 300 kg for every part.
Of course, this proportion was taken out of my ass and may not be in accord with reality, but you must remember that PSI (pound per square inch) and KgfCm² (kilogram force per square centimeter) can be seen as the simple force applied to a certain area.
And as such, you could (probably) take the material characteristics and "translate" it to the maximum force said material would be ale to withstand in the given situation.
If I want this material to withstand 3000 kg, I would need it to be, supposedly, 10 times thicker than one would normally need for a steel material, for example.
(I think)
Steel has a tensile strength of 400 to 550 MPa and a compressive strength of 250 to 400 MPa (for structural steel).
A 5 ton hydraulic cylinder that works at a pressure of 16 MPa (160 bars) has 10mm of wall thickness and weights around 10kg.
Even if I don't actually make an hydraulic cylinder out of HDPE, I would still need to make things with more or less 100mm (10cm) of thickness for structural resilience.
... I think.
------------------------------------------------------------------------------------------------------------------------------
Also, I was thinking on a way of making steel/metal parts in general in a DIY setup and on the cheap. Although I don't know how well I would be successful on such endeavor.
So, the idea would be more or less like this:
1: Make iron oxide
2: Transform the Iron oxide into pure iron powder with the reduction reaction.
3: Mix said powder with the ideal proportion of iron and carbon to generate steel.
4: Place the mixture in a container with little oxygen/air.
5: Place the thermite around this container and in a place away from people.
6: Activate the thermite and melt the metal
7: Transfer the liquid steel to the desired mold.
The idea would be to use the thermite reaction involving iron oxide and pure aluminium powder in order to reach the necessary temperatures.
However, the problem is how to transfer the heat from the reaction to the thing you want to melt without having both liquid metals mixing with each other and making useless slag.
My idea was to use either copper or even sand, since sand is a good thermal "battery", so to speak.
Basically, homemade thermites aren't that hot/powerful, so I thought that maybe the copper would survive, since it would only transfer the heat instead of being the thing that melts.
Plus, some electric kilns work by inserting the electric heating element on the walls of the kiln, heating its insides and then the crucible with the material to be melted. It wouldn't be much different from this idea.
(there are more videos on this series, by the way)
And even then, I doubt I would be able to make pieces with enough precision and strength.
But hey, if you want to do it, go for it.
------------------------------------------------------------------------------------------------------------------------------
Also, dekutree64 just gave an interesting suggestion that I didn't consider while writing this project log: using aluminium.
Aluminium melts at "only" 660 ºC, unlike steel, that needs twice or trice this temperature to be molten and is way easier to handle.
This kiln is way easier to build and use than previously mentioned electric kilns (the guy easily melts aluminium), besides, I would bet that if you take a really buffy microwave, you would be able even melt steel.
But I would try to make an "open kiln", let's say, tearing open a microwave and "glueing" the magnetron to the ceramic wool with the silicon carbide crucible inside of it rather than making all this work for a small crucible that would fit inside a microwave.
You would be able to make something more akin to an actual kiln this way.
And maybe you wouldn't even need to make a silicon carbide kiln, using silicon carbide plates/blocks/pieces like the heating element on conventional electric kilns.
Like this one, he cut/broke a silicon carbide griding disk for the heating element.
------------------------------------------------------------------------------------------------------------------------------
Also, like Dekutree64 mentioned in his comment: you could use scrap aluminium for that.
However, you would need to be careful to actually take aluminium instead of Tin or stainless steel. And you would also need to take off the paint and the plastic inliner on soda cans, the best way I could find was to just heat the can until the plastic and the paint just peel off.
You could do it after cutting everything in little pieces (not powder, pieces) and throghouly washing it until it is relatively clean.
------------------------------------------------------------------------------------------------------------------------------
Although it wouldn't be that stronger than extruded aluminium, you could use a few "cheats" to heat treat it in order to increase its strength.
For example, in this video the guy shows a few heat treatments in order to make aluminium castings stronger. Not to mention that you could add a little bit of silicon metal and/or other materials in order to make something as strong as aerospace aluminium.
------------------------------------------------------------------------------------------------------------------------------
ChatGPT:
"It seems like there might be a slight mix-up in the alloy designations you provided. The common aerospace aluminum alloys are 6061 and 7075, not 7071. Let's take a look at the materials found in these two popular aerospace aluminum alloys:
- Aluminum 6061: Aluminum alloy 6061 is a versatile and widely used material in aerospace applications due to its good combination of strength, workability, and corrosion resistance. It typically contains the following elements:
- Aluminum (Al): The primary element, constituting the majority of the alloy.
- Magnesium (Mg): Typically around 1.0% to 1.2%. It provides strength and increases the alloy's heat-treatability.
- Silicon (Si): Usually around 0.4% to 0.8%. It aids in improving the alloy's casting properties and contributes to strength.
- Iron (Fe): Usually less than 0.7%.
- Copper (Cu): Typically around 0.15% to 0.4%. It enhances strength and corrosion resistance.
- Zinc (Zn): Usually less than 0.25%.
- Titanium (Ti): Sometimes present in trace amounts for grain refinement and control.
- Aluminum 7075: Aluminum alloy 7075 is known for its exceptional strength-to-weight ratio and is commonly used in aerospace and high-performance applications. It typically contains the following elements:
- Aluminum (Al): The primary element, making up the bulk of the alloy composition.
- Zinc (Zn): Typically around 5.1% to 6.1%. It is a significant alloying element that enhances strength.
- Magnesium (Mg): Usually around 2.1% to 2.9%. It further improves the alloy's strength and contributes to its heat-treatability.
- Copper (Cu): Typically around 1.2% to 2.0%. It provides additional strength and hardness.
- Chromium (Cr): Sometimes present in small amounts, aiding in improving corrosion resistance.
Both 6061 and 7075 aluminum alloys are widely used in the aerospace industry for various components, such as aircraft structures, wings, fuselage parts, and more, due to their favorable mechanical properties and lightweight characteristics. The specific composition and processing of these alloys can vary depending on the intended application and desired properties."
------------------------------------------------------------------------------------------------------------------------------
So, in resume, you would need a few grams of each material for your homemade 6061/7075 aluminium alloy.
Also, what defines aluminium 6061 and 7075 grades are more related to its heat treatment rather than its alloying, but you can only heat treat to this extent due to its alloying.
"The tensile strength of pure aluminium is around 90 MPa but this can be increased to over 690 MPa"
Source: https://www.azom.com/article.aspx?ArticleID=2863
(I searched other sources and the compressive strength increases to a similar extent, around 600 MPa)
------------------------------------------------------------------------------------------------------------------------------
"Aluminum alloys 6061 and 7075 are both popular and widely used in various industries due to their excellent mechanical properties and high strength-to-weight ratio. They undergo different heat treatments to enhance their properties, making them suitable for specific applications. Below are the common heat treatments for each alloy:
Heat treatments for Aluminum 6061:
- Annealing (O Temper): This is the softest and most ductile condition of 6061 aluminum. It involves heating the alloy to around 775°F (413°C) and then allowing it to cool slowly in the furnace. This treatment reduces internal stresses and improves workability.
- Solution Heat Treatment (T4 Temper): The alloy is heated to around 990°F (532°C) for a specific period and then quenched rapidly in water. This treatment dissolves the alloying elements and prepares it for precipitation hardening.
- Precipitation Hardening (T6 Temper): After the solution heat treatment, the material is aged at around 320°F to 350°F (160°C to 177°C) for several hours. This results in the precipitation of strengthening phases, such as Mg2Si, which significantly increases the alloy's strength.
- Artificial Aging (T651 Temper): In this process, the material is artificially aged after the solution heat treatment, which results in improved mechanical properties, especially for thicker sections. The aging is typically done at around 350°F (177°C) for a few hours.
Heat treatments for Aluminum 7075:
- Solution Heat Treatment (T6 Temper): Similar to 6061, the 7075 alloy undergoes solution heat treatment by heating it to around 990°F (532°C) and then quenching it rapidly in water. This treatment prepares the alloy for precipitation hardening.
- Precipitation Hardening (T6 Temper): After the solution heat treatment, the material is aged at about 250°F to 300°F (121°C to 149°C) for several hours, leading to the precipitation of strengthening phases like MgZn2. This significantly increases the alloy's strength.
- Artificial Aging (T651 Temper): As with 6061, artificial aging is applied to 7075 to further improve its mechanical properties, especially for thicker sections. The aging is typically done at around 250°F to 300°F (121°C to 149°C) for a few hours.
It's essential to note that the specific heat treatment processes and parameters can vary slightly depending on the manufacturer and application requirements. Proper heat treatment significantly impacts the mechanical properties of these aluminum alloys, allowing engineers to tailor their performance to meet specific design needs."
------------------------------------------------------------------------------------------------------------------------------
I tried to search for glass fiber aluminium composites, but I couldn't find anything besides actually "gluing" fiberglass/carbon fiber with epoxy on alumnium plates.

I could only find a few papers on the subject, such as this one (you may need to use the sci-hub to read the entire article:
https://www.sciencedirect.com/science/article/abs/pii/S0925838819325083
The articles concludes that the composite was stronger than monolithic aluminium parts.
I think this article is also interesting:
One of the metal matrix composites were made using aluminium metal and aluminium oxide (alumina) powder.
By the way, I think that is hard to find fiber glass aluminium composites because the fiber glass needs to be porpusefuly made for high temperatures, such as silica-alumina ceramic wools for kilns/furnaces.
But I don't know if these kinds of fibers would increase the mechanical strength of such composite, after all, that's not the use for such fiber.
Regular fiber glass can only withstand temperatures to a few hundred degrees, so does basalt fiber and carbon fiber. Also, carbon kinda "kills" aluminium metal, so you wouldn't be able to use carbon fiber either (I think).
You could also use Steel wires/cables for the composite, since these have a waaaay higher melting point.
Kinda silly how I didn't think of such thing before.... humm
I found this article here:
https://www.researchgate.net/publication/279284161_Composites_of_Aluminium_Alloy_Matrix_Reinforced_by_a_Steel_Mesh
------------------------------------------------------------------------------------------------------------------------------
You can still use aluminium for a stator, but it is not well suited for high torque applications (accordingly to Chat GPT).
"Aluminum alloys used for stators in electric motors are typically chosen based on their specific mechanical and electrical properties. Some of the commonly used aluminum alloys for stators include:
- Aluminum 3004: This alloy offers good formability and high strength, making it suitable for stator components that require shaping or bending during manufacturing.
- Aluminum 5052: This alloy has excellent corrosion resistance and good weldability. It is often used in stators that may be exposed to harsh environmental conditions.
- Aluminum 6061: This alloy is known for its excellent machinability and good strength-to-weight ratio. It is used in stators that require high mechanical stability.
- Aluminum 6063: This alloy is prized for its extrudability and is often used in applications where intricate shapes or custom profiles are needed.
- Aluminum 7075: This high-strength alloy is used in applications requiring superior mechanical properties and performance under high loads or stresses.
- Aluminum 8000 series alloys: These alloys, including 8011 and 8079, are commonly used in electrical applications due to their excellent electrical conductivity.
The choice of the specific aluminum alloy will depend on factors such as the motor's operating conditions, mechanical requirements, and environmental exposure. Additionally, in some cases, the alloy may be customized with additional elements to achieve specific performance characteristics.
It's important to note that while aluminum alloys offer advantages such as lightweight and good thermal conductivity, they may not be as suitable for high-power or high-torque applications compared to other materials like iron-based alloys (e.g., silicon steel) or soft magnetic composites (SMCs) due to their lower magnetic permeability. Therefore, the selection of the appropriate alloy should align with the specific requirements of the motor design and intended application."
Also, something I think it would be relevant, since one would probably take aluminium from scrapyards, normally in the form of soda cans:
"Soda cans are typically made from aluminum alloys that are specifically designed for easy fabrication, low cost, and good formability. The most common aluminum alloy used for soda cans and many other daily objects is known as 3004 alloy. The composition of 3004 aluminum alloy is as follows:
- Aluminum (Al): 95.8>#/li###
- Magnesium (Mg): 0.8>#/li###
- Manganese (Mn): 1.3>#/li###
- Silicon (Si): 0.3>#/li###
- Iron (Fe): 0.7>#/li###
- Others: 1.1>#/li###
This alloy is part of the 3xxx series of aluminum alloys, which are known for their excellent formability and corrosion resistance. It can be easily shaped into thin sheets to create cans, making it a popular choice for beverage packaging.
When melting aluminum scrap from soda cans or any other aluminum objects, it's essential to ensure the material is clean and free from any contaminants, such as paint, coatings, or other non-aluminum materials, which could affect the quality of the castings. Additionally, it's important to take proper safety precautions when melting aluminum, as it involves high temperatures. Make sure to work in a well-ventilated area, wear appropriate protective gear, and follow proper procedures to prevent accidents and ensure a successful casting process."
------------------------------------------------------------------------------------------------------------------------------
"Titanium Aluminides (TiAl): These intermetallic compounds offer a unique combination of low density and high strength at elevated temperatures, making them useful for aerospace and automotive applications."
You can find titanium dioxide for a really cheap price online (compared to its metallic counterpart), it is normally used as a pigment, basis for makeup, food additive and/or abrasive element.
"The reduction of titanium oxide (TiO2) with hydrogen gas (H2) typically occurs at elevated temperatures in a controlled atmosphere. The process involves removing oxygen from the titanium oxide to produce titanium metal. This reduction reaction is commonly used in the production of titanium and its alloys. The conditions under which this reduction occurs are as follows:
- Temperature: The reduction of titanium oxide with hydrogen gas usually occurs at temperatures in the range of 800°C to 1,200°C (1,472°F to 2,192°F). The specific temperature can vary depending on factors such as the type of titanium oxide being reduced and the desired reaction kinetics.
- Atmosphere: The reduction is typically carried out in a controlled atmosphere of hydrogen gas. The presence of a reducing gas like hydrogen prevents the re-oxidation of the titanium during the process.
- Pressure: The reduction process can be conducted at atmospheric pressure or at slightly elevated pressures, depending on the specific setup and requirements of the production process.
- Reaction Time: The duration of the reduction process can vary based on factors such as the temperature, particle size of the starting materials, and the desired extent of reduction. Generally, the reduction time can range from several hours to several days.
- Catalysts: In some cases, catalysts may be used to enhance the reduction reaction kinetics and lower the required temperature. For example, iron powder or other transition metal powders can act as catalysts in the reduction of titanium oxide.
It's important to note that the reduction of titanium oxide with hydrogen is an energy-intensive process and is typically carried out in specialized equipment such as fluidized bed reactors or vacuum furnaces. The resulting titanium sponge produced from this reduction process is further processed to obtain usable titanium metal or used as a feedstock for producing titanium alloys.
The reduction of titanium oxide is a fundamental step in the production of titanium, which is a critical material in various industries, including aerospace, automotive, medical implants, and more."
Titanium aluminides are a class of intermetallic compounds that consist primarily of titanium and aluminum. They are known for their lightweight, high-temperature strength, and good corrosion resistance, making them valuable for aerospace and other high-performance applications. The exact composition and processing steps can vary depending on the specific alloy and its intended use, but I can provide a general overview of the process.
1. Alloy Composition: The composition of titanium aluminides typically includes a range of elements to achieve the desired properties. In addition to titanium (Ti) and aluminum (Al), other elements such as niobium (Nb), tantalum (Ta), and vanadium (V) may be added to enhance certain characteristics. The specific percentages of each element can vary widely based on the alloy's intended properties.
2. Melting and Alloying: The process of making titanium aluminides generally involves melting the constituent metals together in a controlled environment, such as a vacuum arc furnace or an induction furnace. The steps involved are as follows:
a. Preparation of Raw Materials: High-purity titanium, aluminum, and any other alloying elements are weighed and mixed according to the desired composition.
b. Melting: The raw materials are loaded into a crucible within the furnace. The furnace is then heated to temperatures exceeding the melting points of the metals. Once the metals are molten, they are thoroughly mixed to ensure homogeneity.
c. Alloying: Alloying elements are added to the melt as needed to achieve the desired composition. The melt is stirred or agitated to ensure proper mixing.
3. Casting: After the alloy is properly mixed and homogenized, it can be cast into the desired shape. There are various casting methods that can be used, such as:
a. Ingot Casting: The molten alloy is poured into a mold to solidify into a large ingot. The ingot can later be processed into various forms, such as sheets, bars, or other shapes.
b. Directional Solidification (DS): This technique involves controlled solidification of the alloy in a specific direction, which can lead to improved microstructure and properties.
4. Hot Working: Titanium aluminides often undergo hot working processes to refine the microstructure and improve mechanical properties. Hot working typically involves processes such as:
a. Hot Rolling: The alloy is heated to elevated temperatures and then passed through rolling mills to reduce its thickness and improve its mechanical properties.
b. Hot Forging: The alloy is heated and shaped using mechanical force to achieve the desired form and properties.
5. Heat Treatment: Heat treatment is a critical step to optimize the microstructure and properties of titanium aluminides. This typically involves:
a. Solutionizing: The alloy is heated to a specific temperature to dissolve any precipitates or impurities, followed by rapid cooling to trap the desired microstructure.
b. Aging: The alloy is held at an intermediate temperature to allow controlled precipitation of strengthening phases within the microstructure.
The specific details of each step, including temperatures, times, and cooling rates, can vary depending on the alloy and its intended properties. Researchers and engineers continually work to optimize these parameters to achieve the best balance of mechanical, thermal, and corrosion properties in titanium aluminides.
I apologize for not providing specific percentage ranges in my previous response. The exact composition of titanium aluminides can vary widely depending on the specific alloy and its intended application. However, I can provide you with a general idea of the typical composition ranges for some common titanium aluminide alloys:
- TiAl (Gamma Titanium Aluminide):
- Titanium (Ti): Approximately 40-48>#/li###
- Aluminum (Al): Approximately 52-60>#/li###
- Ti3Al (Alpha-2 Titanium Aluminide):
- Titanium (Ti): Approximately 89-92>#/li###
- Aluminum (Al): Approximately 7-10>#/li###
- Other elements (e.g., trace amounts of oxygen, nitrogen)
- TiAlNb (Titanium Aluminide with Niobium):
- Titanium (Ti): Varies, but often around 45-48>#/li###
- Aluminum (Al): Varies, but often around 51-54>#/li###
- Niobium (Nb): Typically around 2-5>#/li###
- Other elements (e.g., trace amounts of vanadium, iron, etc.)
- TiAlTa (Titanium Aluminide with Tantalum):
- Titanium (Ti): Varies, but often around 40-45>#/li###
- Aluminum (Al): Varies, but often around 50-55>#/li###
- Tantalum (Ta): Typically around 4-8>#/li###
- Other elements (e.g., trace amounts of niobium, chromium, etc.)
- TiAlV (Titanium Aluminide with Vanadium):
- Titanium (Ti): Varies, but often around 35-40>#/li###
- Aluminum (Al): Varies, but often around 58-63>#/li###
- Vanadium (V): Typically around 1-3>#/li###
- Other elements (e.g., trace amounts of niobium, chromium, etc.)
It's important to note that these composition ranges can vary depending on the specific alloy variant, the intended properties, and the processing techniques used. The addition of other elements and trace impurities can further affect the alloy's properties and performance. Engineers and researchers adjust these compositions to achieve the desired balance of strength, ductility, high-temperature stability, and other properties for specific applications.
------------------------------------------------------------------------------------------------------------------------------
Nickel salts are chemical compounds that contain nickel ions (Ni2+) combined with various anions (negatively charged ions). These salts are formed through reactions between nickel and different acids or bases. Nickel salts have a wide range of applications in various industries, including chemical manufacturing, electroplating, pigments, and catalysts. Some common examples of nickel salts include:
- Nickel Sulfate (NiSO4): This is one of the most commonly used nickel salts. It's used in electroplating processes to deposit nickel onto surfaces, as well as in the production of nickel-cadmium batteries and as a precursor for other nickel compounds.
- Nickel Chloride (NiCl2): Nickel chloride is used in electroplating and as a catalyst in chemical reactions. It's also used in the manufacture of certain nickel alloys and as a source of nickel for other compounds.
- Nickel Carbonate (NiCO3): Nickel carbonate is used in the production of nickel-based catalysts, as a precursor for other nickel compounds, and in ceramics and glass manufacturing.
- Nickel Nitrate (Ni(NO3)2): Nickel nitrate is used in nickel plating, as a catalyst in organic reactions, and in the preparation of other nickel compounds.
- Nickel Acetate (Ni(CH3COO)2): Nickel acetate is used in electroplating, as a mordant in textile dyeing, and in certain chemical reactions.
- Nickel Oxide (NiO): While not strictly a "salt" in the traditional sense, nickel oxide is a compound containing nickel and oxygen. It's used as a pigment and in ceramic applications.
These nickel salts and compounds can be sourced from various industrial processes, including the production of metals, pigments, catalysts, and chemicals. They are used in a variety of applications due to the unique properties of nickel, such as its resistance to corrosion, electrical conductivity, and catalytic activity.
When considering the extraction of nickel from salts, it's important to note that the concentration of nickel in these compounds can vary, and the economics and feasibility of extraction depend on factors such as the specific salt, the extraction methods, and the intended end use of the extracted nickel.
The process of converting nickel salts back into pure nickel metal involves a reduction reaction. Reduction is a chemical process in which a compound gains electrons, leading to the removal of the oxygen, chlorine, or other non-metallic elements from the compound. The specific method used to reduce nickel salts to pure nickel metal can vary depending on the type of salt and the desired end product. Here are general outlines of how some common nickel salts can be reduced to obtain pure nickel metal:
- Nickel Sulfate (NiSO4):
Nickel sulfate can be reduced to pure nickel metal using a process called electrolysis. In this method, an electrolytic cell is set up with a nickel sulfate solution as the electrolyte. A direct electric current is passed through the solution, causing the nickel ions (Ni2+) to gain electrons and deposit onto a cathode as pure nickel metal. The reduction reaction at the cathode is:
Ni^2+ + 2e^- → Ni
- Nickel Chloride (NiCl2):
Reduction of nickel chloride can also be achieved through electrolysis. The setup is similar to that of nickel sulfate reduction. During electrolysis, the nickel ions in the solution gain electrons at the cathode and deposit as nickel metal:
Ni^2+ + 2e^- → Ni
- Nickel Carbonate (NiCO3):
Nickel carbonate can be thermally decomposed to produce nickel oxide (NiO) and carbon dioxide (CO2) gas. The nickel oxide can then be further reduced to nickel metal using a suitable reducing agent, such as hydrogen gas (H2) at high temperatures. The reduction reaction is as follows:
NiO + H2 → Ni + H2O
- Nickel Nitrate (Ni(NO3)2):
Nickel nitrate can also undergo thermal decomposition to form nickel oxide (NiO), which can then be reduced to nickel metal using a reducing agent like hydrogen gas:
NiO + H2 → Ni + H2O
It's important to note that the reduction of nickel salts requires careful control of reaction conditions, such as temperature, pressure, and the choice of reducing agent, to ensure efficient and complete conversion to pure nickel metal. Additionally, safety precautions must be taken when working with high temperatures and potentially hazardous chemicals.
The choice of method may depend on factors such as the starting material, the desired purity of the final nickel metal, and the scale of the operation. Always ensure that the reduction process is conducted in compliance with safety guidelines and regulations.
------------------------------------------------------------------------------------------------------------------------------
Inconel and titanium were innacessible materials for me, but with this, now I think it would be possible to not just make inconel and titanium aluminide (both are equally excellent), but that maybe now the turbine/turboshaft engine is now a viable option in a DIY setup.
Even the gears and bearings would be able to withstand such insane conditions...

------------------------------------------------------------------------------------------------------------------------------
Magnesium Reduction:
In this method, silicon oxide (silica) is reduced using magnesium metal as the reducing agent. The reaction takes place at high temperatures in a controlled environment. The magnesium reacts with the oxygen in silicon oxide, forming magnesium oxide and leaving behind pure silicon.
Converting silicon carbide (SiC) to pure silicon involves a process known as carbothermic reduction. Silicon carbide is a compound composed of silicon and carbon, and carbothermic reduction involves using a carbon source to reduce the silicon carbide to elemental silicon. Here's an overview of the carbothermic reduction process for producing pure silicon from silicon carbide:
- Preparation of Silicon Carbide: Silicon carbide is typically produced by reacting silica (sand) and carbon in an electric furnace at high temperatures. The reaction produces silicon carbide crystals. The silicon carbide is then further processed to obtain a suitable feedstock for carbothermic reduction.
- Carbothermic Reduction Reaction:
The carbothermic reduction of silicon carbide involves heating the material in the presence of a carbon source, usually in the form of coke or charcoal. The carbon reacts with the silicon carbide, leading to the reduction of silicon carbide to silicon and carbon monoxide gas:
SiC + 2C → Si + 2CO
- High-Temperature Furnace: The carbothermic reduction reaction is carried out in a high-temperature electric arc furnace or similar equipment. The furnace is heated to temperatures ranging from about 2000°C to 2500°C (3632°F to 4532°F).
- Formation of Molten Silicon: As the reaction progresses, the silicon carbide is reduced, and molten silicon collects at the bottom of the furnace. The carbon monoxide gas is released as a byproduct.
- Separation and Collection: The molten silicon is then collected from the furnace. The collected silicon may be cast into ingots or other forms for further processing.
- Purification and Refining: Similar to the reduction of silicon oxide, the obtained silicon from carbothermic reduction may undergo purification and refining processes to remove impurities and achieve the desired level of purity.
It's important to note that the carbothermic reduction of silicon carbide is an energy-intensive process and requires specialized equipment and facilities. The quality and purity of the final silicon product are critical, especially for applications in the electronics and semiconductor industries.
The choice of method for producing pure silicon depends on factors such as the starting materials, energy efficiency, environmental considerations, and the intended use of the silicon. Careful attention to safety, environmental regulations, and proper handling of materials is essential when carrying out carbothermic reduction or any other industrial process.
------------------------------------------------------------------------------------------------------------------------------
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.
Ooh. Zip chains. I didn't know about zip chains.
(reading because comment activity here came up in the feed)
Are you sure? yes | no
Well, thanks for the comment xD
Are you sure? yes | no
The beauty of aluminum is that it's strong and light by itself so you don't have to mess with composites or elaborate structures. But that is an interesting thought, making a steel/aluminum composite by putting wires in the mold before pouring. Maybe even fine wire all tangled up, similar to the electroplated carbon fiber technique but fully solid https://hackaday.io/project/182026-self-created-metal-composites
But might need vacuum to get the aluminum to fully wet it out. I'll have to try it sometime. Probably still no good for motor stators though. You would get some magnetic iron in there, but also high conductivity so eddy currents would be terrible.
Hmm, maybe tangled soft iron wire cast in epoxy... the convoluted path with small contact areas between wires should resist eddy currents reasonably well.
Are you sure? yes | no
Oh, I didn't knew about this property of aluminium... Interesting...
The iron-wire-resin is a nice idea for the iron stator, but I would advise you to maybe try to do some kind of lamination, let's say, add the iron wire and epoxy, then add a resin layer only using a few ml. Then repeat.
But the problem with soft magnetic composite cores is that air and/or the resin matrix will occupy valuable space that could be used by the iron/silicon steel.
Unfortunately I don't know how to make quality laminations at home... >.>
Maybe using those electric arc and/or microwave kilns to melt iron powder into paper thin laminations without the need for complex and expensive equipment...
Are you sure? yes | no
If eddy currents are still a problem, I'd try coating the wire with shellac first rather than the labor-intensive laminations. But yes, this approach will always be inferior due to space occupied by resin. And wire would probably be more expensive by weight than proper silicon steel sheet anyway. But at least you could use all of it, whereas sheet produces a lot of (recyclable) waste.
Are you sure? yes | no
Collect scrap aluminum and cast it in sand or plaster molds. Not as strong as extruded aluminum due to the crystal structure formed when cooling in the mold, and hydrogen bubbles from absorbed water vapor when melted, but for the price and simplicity it's hard to beat. Small and detailed things need vacuum casting because the surface tension of the liquid metal tends to keep it from filling tight spots in the mold, but if you design with more rounded shapes then a simple pour should do it.
Thermal shrinkage is tricky to compensate for if you need precise dimensions. Ideally you just make it a little oversized and then machine the critical features. But it would probably also work to cast undersize and build it up with J.B. Weld (or maybe even Bondo for really cheap), like to fit parts to the inside/outside diameters of ball bearings. Wash with soap and warm water before trying to bond anything to aluminum. Any microscopic film of oil and it won't stick worth a darn. But intentionally rub some oil on the bearing to prevent the epoxy from bonding to it, so you can get it back apart later.
Aluminum brazing is another great technique to learn. Almost like wood glue for metal :) I struggled at first with some brazing rods I bought on ebay (seem to have too high melting temperature), but my dad had some that melt at 732F and that makes it easy. All you need is a little propane torch.
Are you sure? yes | no
Oh yeah, I forgot about aluminium, thanks for the suggestion :)
Are you sure? yes | no