Give me (more) fuel, give me (more) fire ...
Same as method #16 but with more powerful heating element.
First more heat
Now we can boil water in seconds
Also made samples with exposed bonding wires.
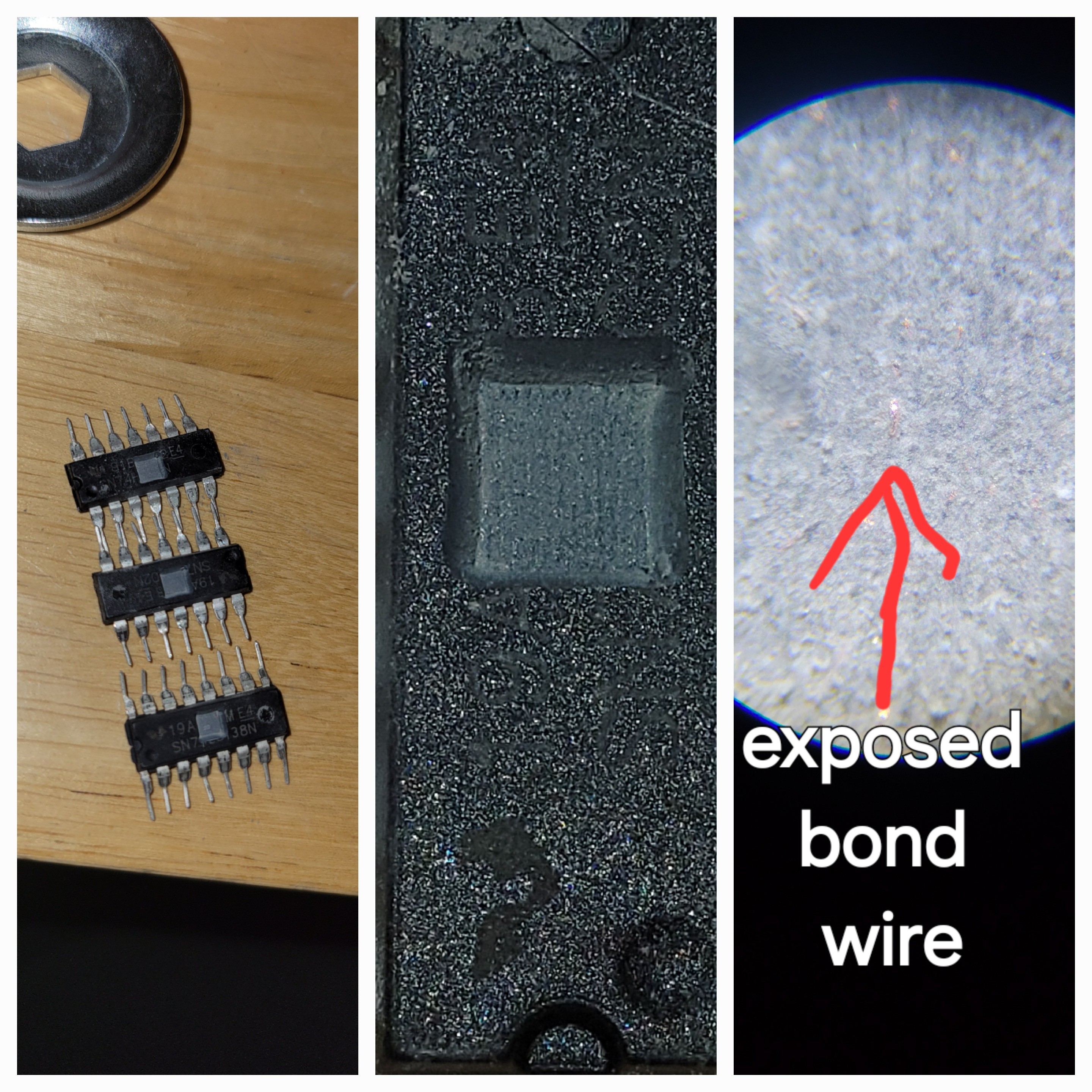
It will look like this

Some refactoring needed as the stove much wider plus I'll need to cover interior with aluminum foil to better distribute heat.(I don't want cardboard to catch fire)
It's always good idea to run dry test.
Compared to the old method the water started to condense almost immediately. The problem it was so hot the hot glue started to melt. I guess I need to run it on lower temperature
Things I did to reduce overheating:
* Reduced power from 5 to 2 on the stove.
* Used iced water
* Covered inside with aluminum foil to spread the heat evenly

Plus, I am using magnetic strips to open and close the box (And holding Nitric acid, we don't want it to fall on someone's head). Made of old 3D printer magnetic surface.
Run dry test, on water the water started to condense after few minutes and dropes were dropping in intervals of 3-5 seconds. The flask's arm was cold as I've hoped.
Now it's time to run real test...
I've started just like the dry test but nothing happened. Added heat insulation and drops started to appear. Got yellow liquid with terrible smell (yellow thing disappeared after few minutes). Once I stopped stove and let it cool down a bit, I've noticed there is rain outside. Some drops must go into Nitric acid :( but I had no choice but to continue.
The reaction was similar to one I saw earlier on method #16.
After some drops were maid I've cooled off everything and noticed I got too much acid. It took me forever to kill it with sodium bicarbonate.
But it was kind of fun
I still don't know if it failed because of drops of water or something else. I will re-run this experiment once never ending rain will stop.
Next time I will use much less Sulfuric acid. Plus I will try to put more potassium nitrate than Sulfuric acid. I have a suspicion that some of Sulfuric acid gets to output.
The result was successful (relatively), I've added first Potassium nitrate and then a little bit of Nitric acid. Everything was the same, except the color of acid was much more yellow

Although I put much less Sulfuric acid I got quite a lot of Nitric acid. (Almost same amount) after 30 minutes of work.
This time it reacted violently with terminals setting red fumes everywhere.
I was able to decap easily with only few drops of Nitric acid.

But again there is no bond wires, maybe because it used hot surface.
So next time I've tried to run with lower temperature. With lower temperature the epoxy turn to material felt like mud. I guess I've ruined the bond wires while trying to dig the 'mud'.
Another test I am running is using Nitric acid at room temperature. After 20 hours nothing happened.
Maybe the temperature was too high. I didn't see the green color that usually shows when copper bond wires dissolve. (When lead frame terminals dissolve there is red color).
I've put my acid on copper wire after first encounter it turned green when more acid added nothing happened, so it must be because a corrosion layer.

Also, checked with fluck, got 10 Ohm instead of 0.2 Ohm.
Looks like we are on the right track.
Then I've noticed something strange
I guess I knew it but ignored it. The hot plate is too hot for Nitric acid. It's just vaporized without doing anything useful, thus process is not efficient. Furthermore, this high temperature might brake bonding wire's connection.
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.