-
1Part 1: Flow Cell using a PH gradient
This flow cell generates electricity by differences in electrode potential in manganese dioxide over a PH gradient, like a fuel cell that uses acids and bases instead of oxygen and fuel. This type of technology, albeit probably with different electrode materials optimized for the task, is being investigated for use in concentration gradient cells for collecting power from salinity gradients at the mouths of rivers as they feed into the ocean.
To build one, you just need to separate two bodies of water with a membrane which restricts the diffusion rate, then place the two electrodes on each side of the membrane, as close together to each other as possible. In the above paper and in our implementation, this is done with carbon fabric and a Celgard 3501 nonselective membrane held close together by a frame for mechanical reinforcement. In the paper this was chosen because it is more cost-effective and robust than ion-exchange membranes used for the same application, at the cost of some efficiency. If you do choose to use an ion exchange membrane instead of Celgard, the rest of the instructions are the same, just make sure it never dries out once assembled, or it may deform and tear or form microcracks.
The container for the bodies of water can be any shape, size or configuration as long as the membrane and electrode materials are cut to size and the electrodes can be electrically contacted (ideally in a way that keeps the electrical contacts from getting wet with the electrolytes, which may corrode the contacts). For best results, the membrane and electrode stack should seal well to prevent any leaks between the two bodies of water from going around their edge: in our implementation we do this with silicone. We have provided some 3D models to construct the following example system:
This device holds some acidic and basic water in two compartments, with a separator in between them. The carbon fabric electrodes have very different electrode potentials in the two solutions, and the membrane allows ion conduction, producing power in a manner similar to a fuel cell. The preparation is simple, but with a few nuances worth mentioning:- Prepare the electrodes
- The electrode ink recipe is the same as described in part 3 of these instructions. It may be easier to prepare in larger batch sizes depending on the sensitivity of your weighing scale. The ink needs to be shaken before each use, making sure to thoroughly break up any clumps. Also make sure you store the ink in a container that will not allow the acetone to evaporate over time, and/or mark the acetone level with a pen for long-term storage so the ink can be reconstituted.
- Cut the conductive carbon fabric to size. For our system that ends up being 20mm by 20mm square, but you'll want to leave a tag roughly 5mm by 5mm above the main electrode to serve as an electrical contact. It may be easier to cut the carbon fabric at 45 degrees to the way it is cut when received, because this helps prevent fraying which can lead to shorting of the electrodes in later steps in the assembly.
- Seal the electrical contact tag: once wet the carbon fabric can wick water up to the electrical contacts, where evaporation and corrosion can produce a false signal. To avoid this we apply a few drops of superglue at the interface between the 5 by 5 mm tag and the 20 by 20 mm electrode surface. This ensures the capillaries between the threads are all sealed up, preventing wicking.
- Shake the ink thoroughly before applying it to homogenize, then with a pipette or eye dropper, apply 4-5 drops per 20mmX20mm electrode surface and spread it to coat evenly. Then allow the freshly prepared electrode to dry.
The process should look something like this:
- Assemble the remaining components
- The separator membrane should be cut slightly wider than the electrodes to help prevent shorting between the electrodes. If the electrodes are a little too big, you may want to trim them and stack the separator and electrodes to check that the separator
- 3D print the provided frame and container files.
- Assemble the cell
- Stack the cell components: frame, electrode, separator, electrode and frame.
- Carefully
apply a small amount of silicone around the edge of the assembly, avoiding the electrical contacts. Make sure not to get any silicone on the exposed electrode surfaces. It may be prudent to allow this assembly to dry for a few hours before proceeding, so that it holds together when being inserted into the container, but it isn't so dry that the silicone has the chance to delaminate when sheared. - Apply a bead of silicone to the groove in the container.
- Insert the frame assembly into the groove in the container.
- Allow the assembly to dry for 1 hour then test for water tightness (the electrodes will initially be hydrophobic). If there is a leak, add more silicone where needed. It is better to do this before the other silicone dries completely, to ensure a strong mechanical joining.
- Allow the silicone to fully dry overnight.
- Apply a small drop of dish soap to each electrode. This will get the carbon fabric electrodes to wick water when the acid and base solutions are added.
- Add Solution and measure results
- Prepare two solutions, one of sodium hydroxide and one of acetic acid, both at 1 M concentration. You will want approximately 20-25 mL of each solution
- Carefully use a pipette to fill each side of the flow cell with different solutions.
- Allow the electrodes to soak up the solution.
- Attach alligator clips to each of the electrodes and measure the voltage
- Prepare the electrodes
-
2Part 1B: Alternitive options
For the pH flow cell, other substances besides sodium hydroxide and acetic acid can be used. The article linked at the beginning of the instructions explains how to use waste carbon dioxide to produce a pH difference in the solutions. Other options would include any solutions with a pH difference that do not damage the other components.
-
3Part 2: Materials Testing and Proof-of-Concept
Though you do not need to build the proof-of-concept device in order to make the flow cell in part 3, we do recommend that if you want to thoroughly validate the effect observed, you go through the materials testing process for all of your materials, even if they are the same type listed here. This can eliminate any materials that could interact with the solutions used in this device and find possible sources of contamination. This is also useful if you want to construct a similar flow cell out of different materials
- Preparing solutions
When preparing the solutions, avoiding contamination is imperative. Wash your gloves first in water, rinsing off the coating, and then in deionized water, to remove any contaminants. Even powder-free gloves have a coating on them. All solutions should be prepared with deionized water, and the materials should be the purest version you can get.
There are four different solutions tested: Ammonium Chloride, Sodium Chloride, Urea Borate, and Urea Monochloride. All the solutions are at the same concentration of 0.001 M. You should make at least 30 mL per sample tested of each solution, with an additional 50 mL to set aside as a control. This will give you enough solution to test each for signs of reactions or contamination.
Preparing solutions at the low concentration of 0.001 M means measuring out materials at a fraction of a gram. If you do not have a scale sensitive enough for this, prepare a more concentrated solution and dilute as needed. These concentrated solutions can be used to make the solutions for the second soaking if they are kept sealed and free of contamination.
For the ammonium chloride and sodium chloride, it is sufficient to add the materials and mix until dissolved. It is important to get pure sodium chloride, as most table salts have additives like iodine. The preparation for both urea compounds is more time-intensive, as they need time to react fully before use. The urea borate and urea monochloride are made by mixing urea with boric acid, and urea with hydrochloric acid, respectively. The goal is to reach a neutral PH.
For making urea monochloride, start by making a solution of hydrochloric acid at the desired concentration. Because hydrochloric acid is a much stronger acid, more urea will be needed to neutralize it. Expect to need to add an order of magnitude more urea than HCl. Once prepared, add urea to the HCl solution. Start by adding 10x as much urea as you did HCl. With that amount of urea added, the reaction will start. It’s endothermic, so the solution gets cold. Use a hot plate to bring the solution to 80 C and allow it to cool. Be sure to cover the solution and add back any water lost during heating. Once the solution is back at room temperature, measure the PH. It will likely still be acidic at this point. Repeat the heating process, adding urea each time, until you get a PH at or near 7. Let the solution sit overnight, as it may continue reacting. Re-measure the PH and if it is still around 7, the solution is ready for testing. If not adjust as needed. As a note: it is better to have an excess of urea than to have unreacted acid but try not to significantly overshoot.
The urea borate solution follows the same procedure as the urea monochloride solution, with the only difference being much less urea is needed. Boric acid is much weaker, so start with 5x as much urea as boric acid, and it to be much closer to neutral after the first round of heating. - Preparing Samples
Each material used in the final device needs to be tested for potential reactivity and contamination. Every material is tested in each of the four prepared solutions, and the results will impact which materials and solutions will be used in the final device.
Start by taking four small samples of each material and putting them into separate glass containers. Make sure the seal on the container is tight, and the lid does not have any metal components. Samples should be in the condition they’ll be when used in the device (fully cured, run through the printer, etc.). You’ll need at least 4 extra empty containers to use as a control, but it’s best to have 8, two for each type of solution, as backup.
Fill one container of each different sample with one of the solutions, so you have all combinations of solutions and samples prepared. Each sample should be in at least 30 mL of solution, in order to have enough for testing. Once the samples are prepared, set them aside for at least a week, allowing any reactions to take place. - Measurements
You’ll make different measurements based on the materials and solution for each sample. The PH will be measured for all samples, chloride ions will be measured for all samples except the ones in urea borate, and ammonia will only be measured for the samples in ammonium chloride. Manganese will only be measured in the samples of manganese material.
For all the samples, first measure the PH of each uncontaminated solution (the containers set aside without materials added). This will be your base PH to measure against. Be sure to rinse the PH probe with deionized water between each of the measurements to prevent cross-contamination.
Once the base PH is established, measure the PH for each of the samples made. The further they are from the base PH, the more likely it is that the material reacted with the solution. Any reaction is unfavorable, so, if at all possible, eliminate use of any material shown to have reacted.
The same procedure is followed for the chloride test, except you do not need to test the urea borate, as it does not contain chlorine. Establish a base level for each set of samples and compare them to the levels tested. The ammonia test is done in the same way, but only for the ammonium chloride samples. Manganese tests need only be run on samples containing the manganese materials. - Second Soak
Once the materials have all been tested, drain the remaining solution and refill with fresh solution. Be sure to use the same solution the sample was initially soaked in. Allow it to soak for an additional week and repeat the measurements. Some materials won’t react during the second soaking, and those materials will be fine to use after being soaked. - Choosing Materials
When deciding which solution and material to use for the final devices, you want to look for ones that do not show significant changes in any of the measurements from the base test to the materials test. Our results showed the materials were best used with the urea borate solution, so that is what the instructions will reflect. If you choose to use a different solution, substitute further mentions of urea borate with your chosen solution. - Assembling Proof-of-Concept Device
- Preparing Components
Components for the proof-of-concept device found in the files section. There are four pieces: the inner cylinder, the outer cylinder, the lid, and the base. All the pieces were designed to be printed successfully without need for supports, as supports for the inner and outer cylinders in particular are difficult to remove .
Once printed, each piece should be handled with rinsed gloves, following the same precautions as seen in step 1. This is to prevent skin oils and other contaminants from getting on the parts. All parts, along with the paraffin wax that will be used to seal them, should be soaked in the urea borate solution. We found this solution to be the best option for this experiment based on the materials chosen. Other solutions may be used depending on the materials and their potential reactions.
Once soaked for a minimum of two days (though a week is preferable), The components and wax can be removed from the solution. Remember to wear rinsed gloves when handling all materials for this experiment. The assembly surface should be rinsed with deionized water to remove contaminants. It should be made of a material like silicone or glass, which won’t transfer any contaminants with contact. - Assembly
To assemble the device, first cut both anion and cation membranes into a rectangle that’s 30 mm by 110 mm. You should then layer them together, with the thicker plastic backing on the outside. They should stick when pressed together, if they don’t then one is likely backwards and should be flipped around.
Once you have the bipolar membrane layered, remove both plastic backings, and wrap it tightly around the inner cylinder. There should be enough for a small overlap at the seam. This overlap needs to line up with the side of the cylinder that is solid, with only one small hole.
Open the outer cylinder and carefully slide the inner cylinder with the membranes into the space. There should be enough flex in the outer cylinder to give clearance to the inner one, so the membranes don’t get caught and fold or tear. Line up the seam on the membranes with the seam on the outer cylinder, so the spokes on the inner and outer cylinders line up as well.
Melt the paraffin wax in a clean glass container that was rinsed with deionized water. Cover the bottom in a thin layer of melted wax, then put in the cylinder assembly. Line the cylinders up with the grooves on the bottom and push it on fully. Do the same with the cap, allowing the wax to cool slightly so it does not run in to the device. Use a small amount of wax to seal the seam of the outer cylinder. - Second Soak
After the device is assembled, it needs to be soaked in a dilute sodium hydroxide solution to activate the membranes. Use a pipette to fill the device with a 0.005 M sodium hydroxide solution, alternating between the inner and outer sections until completely full. Lightly shake and tap the device to make sure there is no trapped air. At this point you can also check for leaks, and use more wax if needed to repair any leaking sections. You should not fill the holes in the lid during the soaking process, they will be capped after the device is filled with the final solution.
Once the device is full, place it in a glass container filled with the same 0.005 M sodium hydroxide solution. - Filling and Sealing
Empty the sodium hydroxide solution from the cylinder. Rinse the cylinder using the urea borate solution. Take a pH measurement of your chosen solution before filling both chambers of the device. Lightly shake and tap the device to remove any trapped air, then carefully cap the holes in the lid with more wax.
- Preparing Components
- Measurements
In order to check that the device works, and prove the proof-of-concept, you need to measure the pH in both chambers after the device is allowed to sit for a few hours to a day. With the device working as expected, you should see a change in the pH between the chambers, where no pH gradient was when filling them.
To measure the pH, use a syringe or pipette to remove the liquid from both chambers, making sure to alternate between the chambers so the liquid level is never significantly higher in one than in the other. Use two different pipettes for the chambers to prevent cross-contamination. Put each sample into a separate container and measure the pH to see the difference. We use a Hanna Instruments Professional Portable Food pH Meter, calibrated with millesimal buffer solutions.
- Preparing solutions
-
4Part 2B: Alternative Options
For the proof-of-concept device, there are a few alternative options that can be explored. In the files you will find the POC Alternative components. These parts are similar to the main components, and would be assembled in the same way. This alternative device is larger, and will use a larger amount of the membrane, but the pH can be measured directly in the device with the pH meter. This allows you to get multiple readings from the solution over time, but comes with the cost of a higher potential for contamination. When not being measured, the holes in the lid should be covered with parafilm or a paraffin wax.
-
5Part 3: Bipolar Membrane Flow Cell
The Bipolar Membrane Fuel Cell is designed to take the concepts shown in Parts 1and 2 and apply them to a small battery-like device that outputs minute amounts of power. It should be noted that gloves need to be worn for this, and the work area needs to be clean and free from contaminants. The bipolar membrane device is sensitive to introduced compounds, especially the salts and oils on your skin. If care isn’t taken, it can invalidate the results. All solutions should be prepared with deionized water, distilled or even reverse osmosis water would introduce contamination that would interfere with the results.
- Download and Print Parts for Bipolar Membrane Device
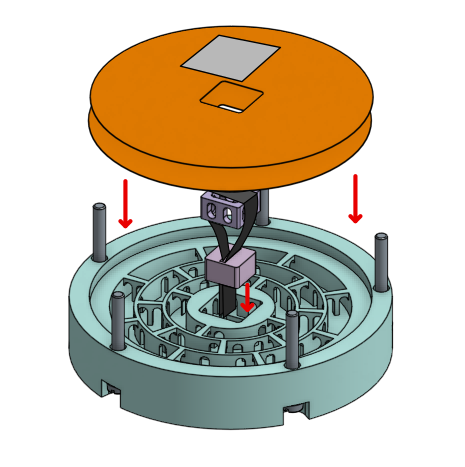
There are four different components used in the bipolar membrane fuel cell. All four can be found in the files section. You will need one top chamber and one bottom chamber, and two electrode frames and chamber caps.
When printing the parts, we used clear PETG. The plastic used is important for this project, it needs to be both impermeable and unreactive. From the testing described in part 2, the clear PETG was the best material found for this project.
We printed on an Original Prusa i3 MK3 printer.
We recommend printing the top and bottom chambers with the interior sections facing upward and supports on the build plate only. It is difficult to remove supports from the interior of the chamber. The electrode frames should be printed with a brim, and if all pieces are printed together the settings should be changed to have both an outer and inner brim. - Electrode Prep
In order to measure the output for the bipolar membrane device, we use an electrode. Prep for this begins with the electrode material. You first need to make manganese dioxide ink to put on the carbon fabric.
To make the ink, in a small, sealable container dissolve 0.1g clear ABS plastic into 20ml of acetone. Once the ABS is fully dissolved, add 0.7g of manganese dioxide (MnO2) and 0.2g of carbon black. Seal the container and shake for 5 seconds. Place into an ultrasonic cleaner for 800s to ensure all the particles are broken down and combined to form a homogeneous mixture. Each fuel cell only uses .5-1 ml of ink, so many can be made with a single batch of ink.
While the ink is mixing, cut the carbon fabric into strips 7mm wide and 70 mm long. It does not need to be exact; the important part is that it is thin enough to fit through the chamber caps, and it is long enough to attach the alligator clamp to for measuring the output when it is wrapped around the electrode frame. You will need 2 electrodes for each battery, though we recommend preparing 3 or 4, in case they rip while being inserted.
Using a pipette, put approximately 15-20 drops of ink onto each carbon fabric electrode, spacing them along the entire length of the fabric. The fabric should be soaked completely when done. Set aside the finished electrodes to dry before putting them in the frame. - Electrode Assembly
The electrode assembly consists of one of the prepared strips of carbon fabric. This should be wrapped around the electrode frame, along the bottom and up the sides. The two tail ends sticking up should be about equal in length. Bring these ends together and feed it through the chamber cap. Pull the chamber cap all the way down the strips until it is sitting directly on top of the electrode frame. Refer to step 4 for a diagram of what this will look like.
Once the electrode is situated within the electrode frame and cap, use superglue along the opening of the chamber cap, on the side of the tail ends. This will serve two purposes, fixing the electrode assembly in place, and preventing evaporation of the solution through wicking.
When both electrode assemblies are dry, insert them into the chambers. Do this by placing the assembly through the center hole of each chamber, with the chamber cap facing outwards. The electrode frame should sit flush with the center surface of the chamber. - Membrane Layering
![]()
There are three sheets of material used in the membrane assembly: the anion membrane, the cation membrane, and the separation membrane. The membrane assembly will be sandwiched between the top and bottom chamber.
It does not matter what order the anion and cation membranes go into the chamber, but we recommend making a note of which one is on which side, especially if you plan on making more than one device. This will allow for better comparison.
The membrane assembly can be done directly in the bottom chamber. Carefully cut each membrane into a circle 65mm in diameter, and then in the center of that, cut a square 16mm on each side. This should result in a membrane that fits into the bottom chamber, overlapping the edges and having an opening around the electrode frame area.
Cut a Celgard 3501 nonselective membrane into a square 20mm on each side. This will be used as a separation membrane and put in the center of the fuel cell to separate the electrodes. It should lay over the holes cut into the anion and cation membranes. - Chamber Assembly
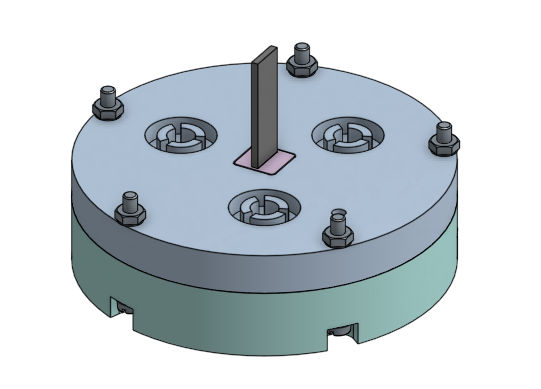
Once the top and bottom chambers are prepared with the electrodes, and the membranes are situated in the bottom chamber, the entire device can be assembled. Holding the electrode assembly on the outside so it doesn’t fall, carefully place the top chamber over the bottom chamber, being sure to line up the bolt holes on the outside of the chambers.
Feed the nylon bolts through the holes and tighten the nuts. Be careful not to over-tighten, as the bolts are soft. - Initial Soak
Once the chamber is assembled, before sealing the chamber, it needs to be filled and soaked in dilute sodium hydroxide. Submerse the chamber in a glass container of 0.005M sodium hydroxide solution. There are holes in each side of the chamber to allow the air to escape and the solution to enter. Flip the chamber around to make sure both sides are filled.
If there are bubbles in the chamber, use a pipette to force solution through the holes to help fill the chamber. Once the chamber is full, allow it to soak for a day. Use a safe material like glass to weigh the chamber down or flip the chamber a few times over the course of soaking in order to fully soak all parts of the chamber. - Drain and Rinse
After soaking, fully drain the chamber. This may require forcing air into the chamber through the holes. Shake it around to get as much liquid out as possible. Do not, however, leave the chamber to dry out, as this could damage the membranes.
When as much of the sodium hydroxide solution as possible has been removed, rinse the interior of the chamber with urea borate solution. Use a pipette to force solution through both sides of the chamber. Once the chamber has been thoroughly flushed, fill it completely and let it sit in a urea borate solution for at least an hour. - Fill and Seal
![]()
Once the chamber has been soaked in the urea borate, drain the chamber, and dry the outside. Melt some paraffin wax and seal along the outside edge of the chamber, as well as around the chamber cap and electrode. Do not seal the holes in the top and bottom yet. Use a pipette to fill one side of the chamber with fresh solution. Remove any air bubbles by tapping the chamber lightly on the table surface, and make sure it is filled to the top. Work quickly as the solution will begin filtering through the membranes. Seal the holes on that side of the chamber with some paraffin wax and repeat on the other side. Once both sides are filled, check for leaks and seal with wax. - Measure Results
Once the chamber is fully sealed, you need to wait for the chamber to equilibrate before measuring the results. This should take 1-3 hours. We used a Keithley DMM6500 multimeter to record the results in real time with high accuracy. While this is very nice, not everyone has access to such an expensive instrument. While the data won’t be as in-depth, you can measure the voltage across a resistor.
- Download and Print Parts for Bipolar Membrane Device
-
6Part 3B: Alternative Options
For the bipolar membrane flow cell, one thing to try is instead of having a hole in the center of both membranes, have only one of the membranes have that hole. Leave the other membrane intact and use that to separate the electrodes instead of the Celgard 3501 nonselective membrane as a separation membrane.
 Michael Perrone
Michael Perrone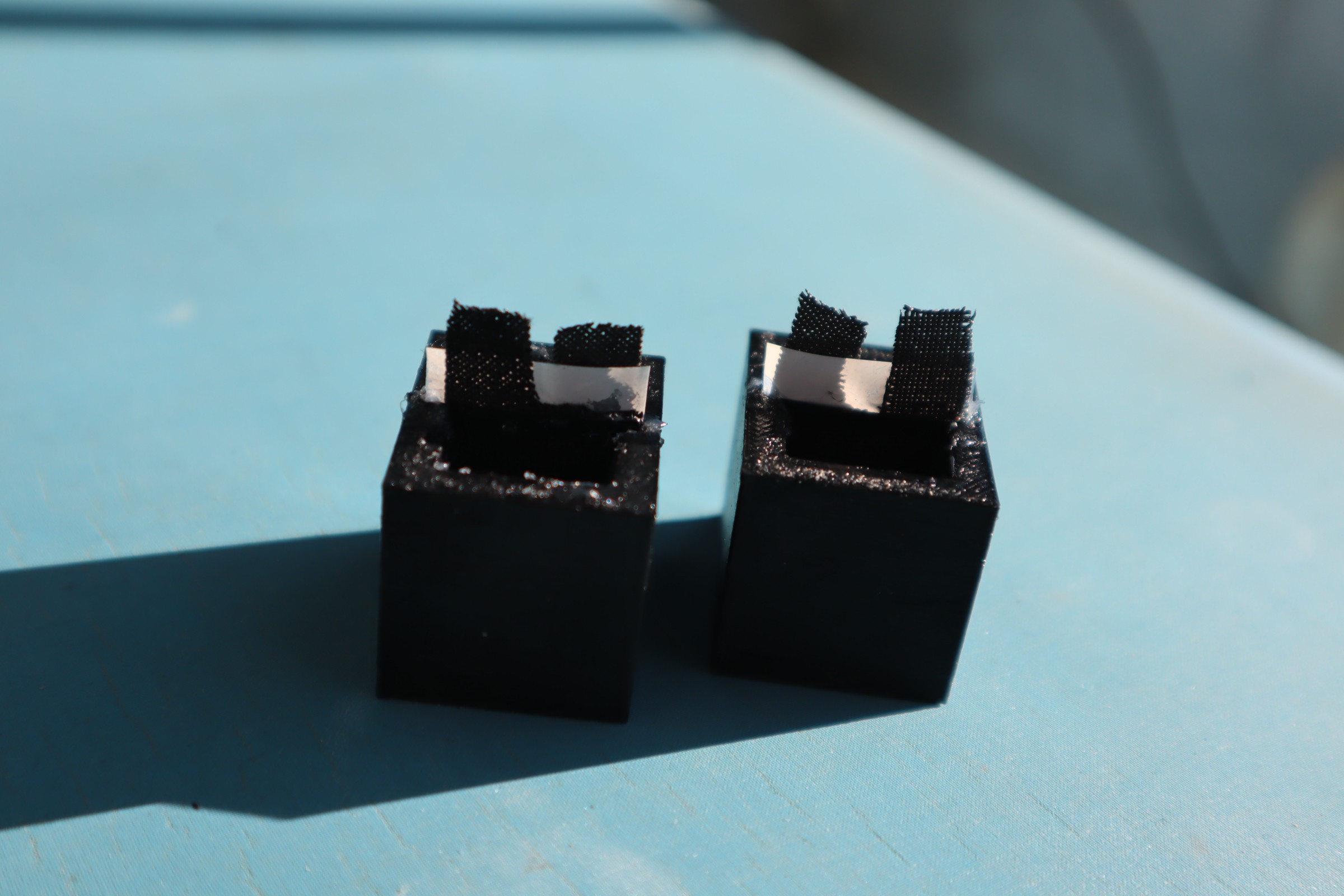

Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.