This project is really just a 'wrapper' for the linear ccd module so for details about driving and reading the TCD1304 refer to that project.
The following is a (very) brief introduction to reaction rates.
For a given reaction:
The reaction rate, v, is defined as:
many reactions have rates that follow the concentration of the reactants as:
However, this is a pure empirical relation and the reaction orders of A and B (n and m) cannot be deduced except by experiment (and to make matters worse, they may change with the conditions of the reaction).
When combining the two expressions for v, we get this differential equation:
Obviously we must ignore B (in the lab this is done by keeping its concentration constant). The reaction order for A, n, usually takes the integral values: 0, 1 or 2 (but anything is possible). For the three "normal" values of n, the differential equation has the following solutions:
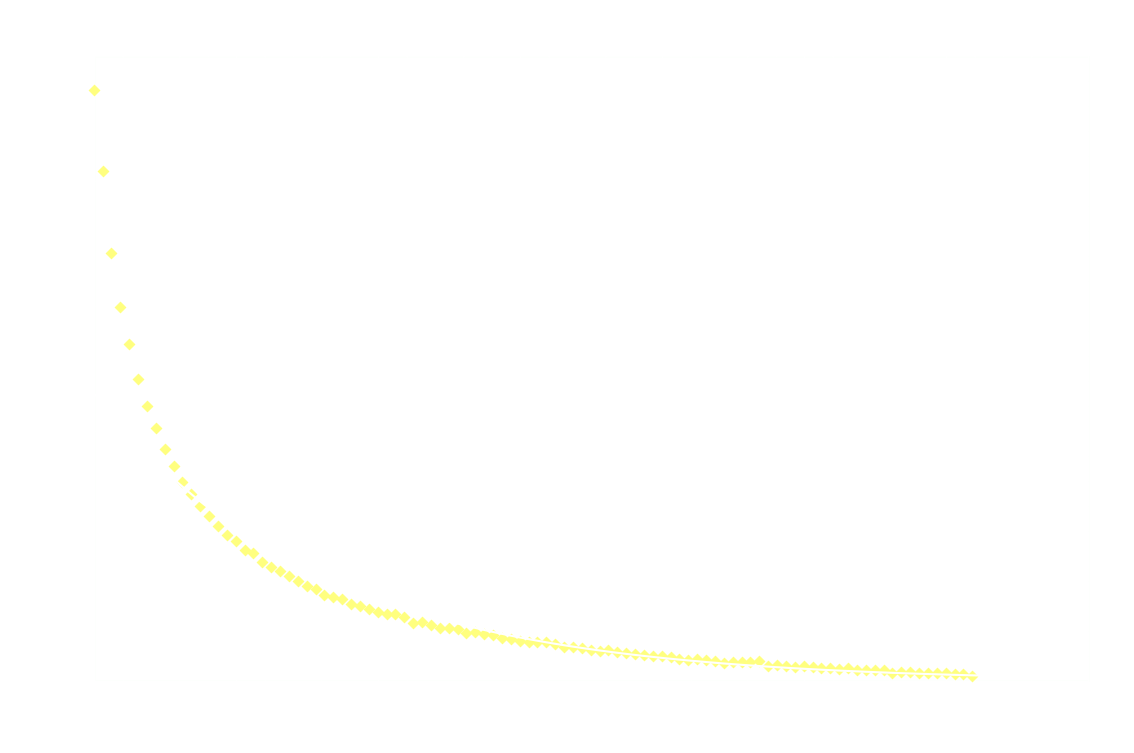
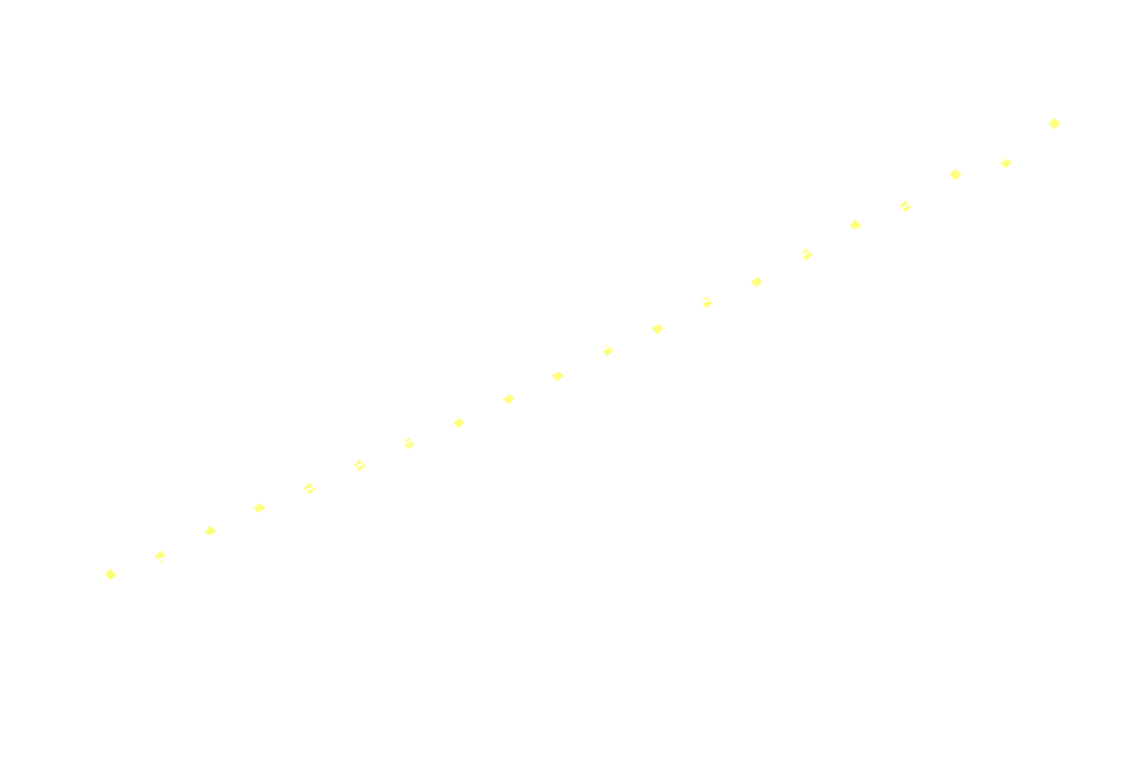
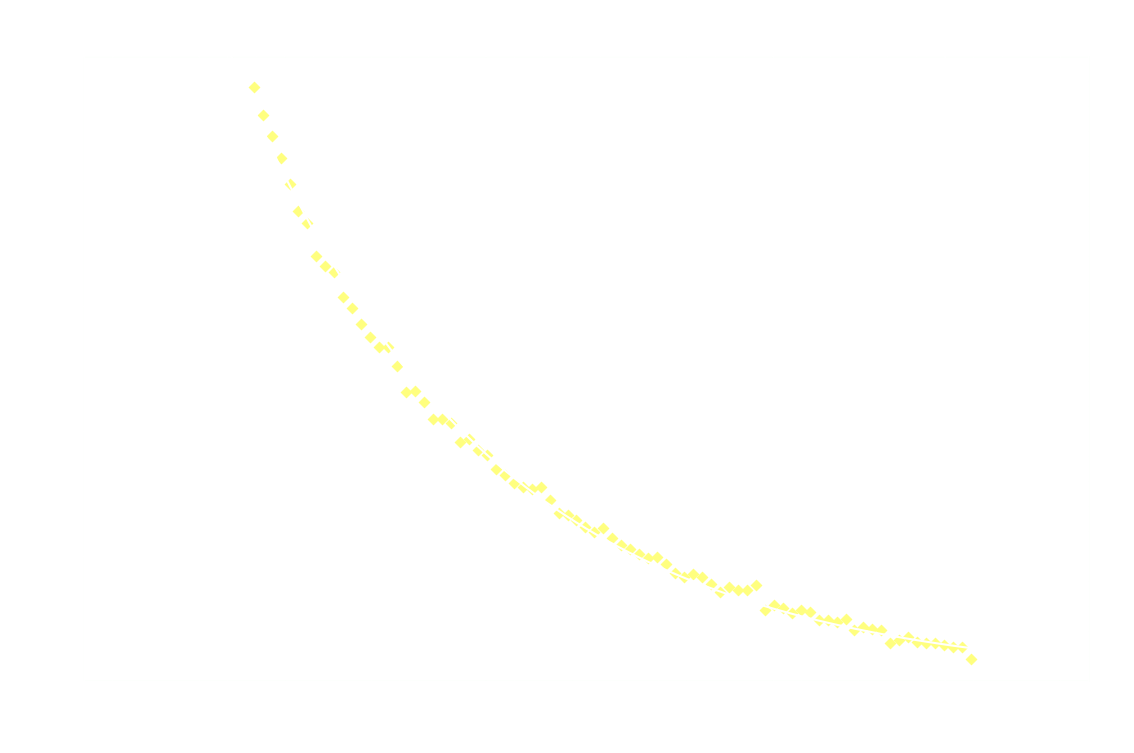
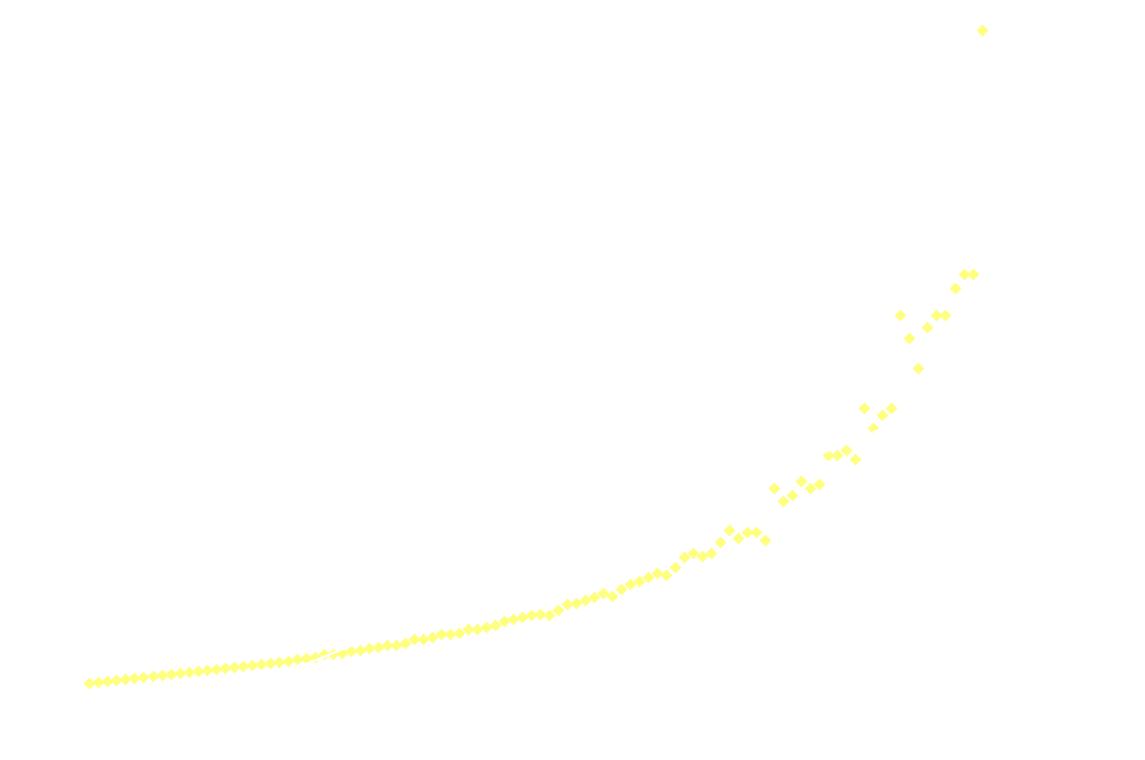
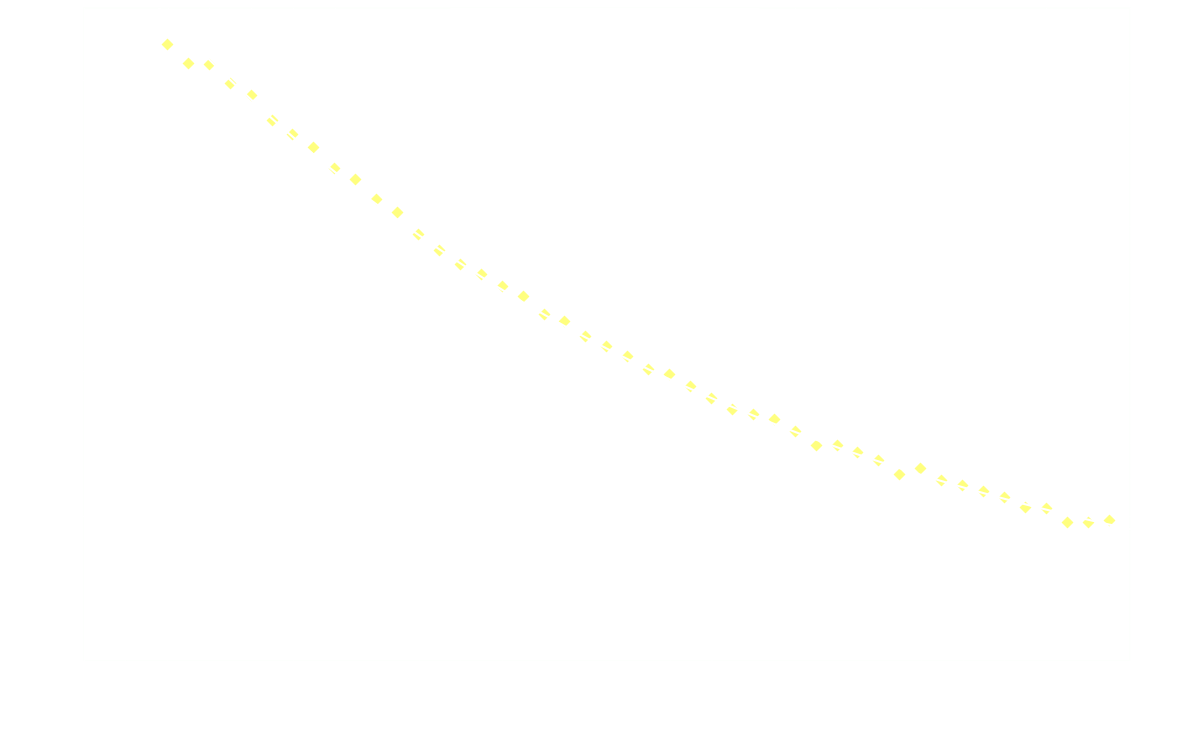
In a chemiluminescent reaction the light intensity, I, is proportional to the reaction rate so we can write:
By measuring I over time, one can essentially find a function that describes the reaction rate as function of time. If the reaction is "well behaved" ie. if the reaction order is either 0, 1 or 2, this function should be identical to the 1st derivative of one of the 3 solutions to the differential equation.
In brief, the reaction rate is:
- constant, for 0th order reactions
- an exponential function of time, for 1st order
- a hyperbola or something (1/t²), for 2nd order
 esben rossel
esben rossel



 Place it over the rpi:
Place it over the rpi:
 Install the nucleo:
Install the nucleo:


 Bruce Land
Bruce Land

 artist_edge
artist_edge
 Tim
Tim
Ha, this is certainly an interesting use of the TCD104 :)