-
Glow in the dark star (ZnS:Cu)
03/23/2018 at 10:54 • 0 commentsGlow in the dark stars contain a phosphorescent pigment consisting of copper doped zinc sulfide (ZnS:Cu) or europium doped strontium aluminate (SrAl2O₄:Eu).
It's super-easy to produce zinc sulfide, one simply mixes solutions of sodium sulfide and a soluble zinc salt e.g. zinc chloride:Zn²⁺ + S²⁻ → ZnS(s)
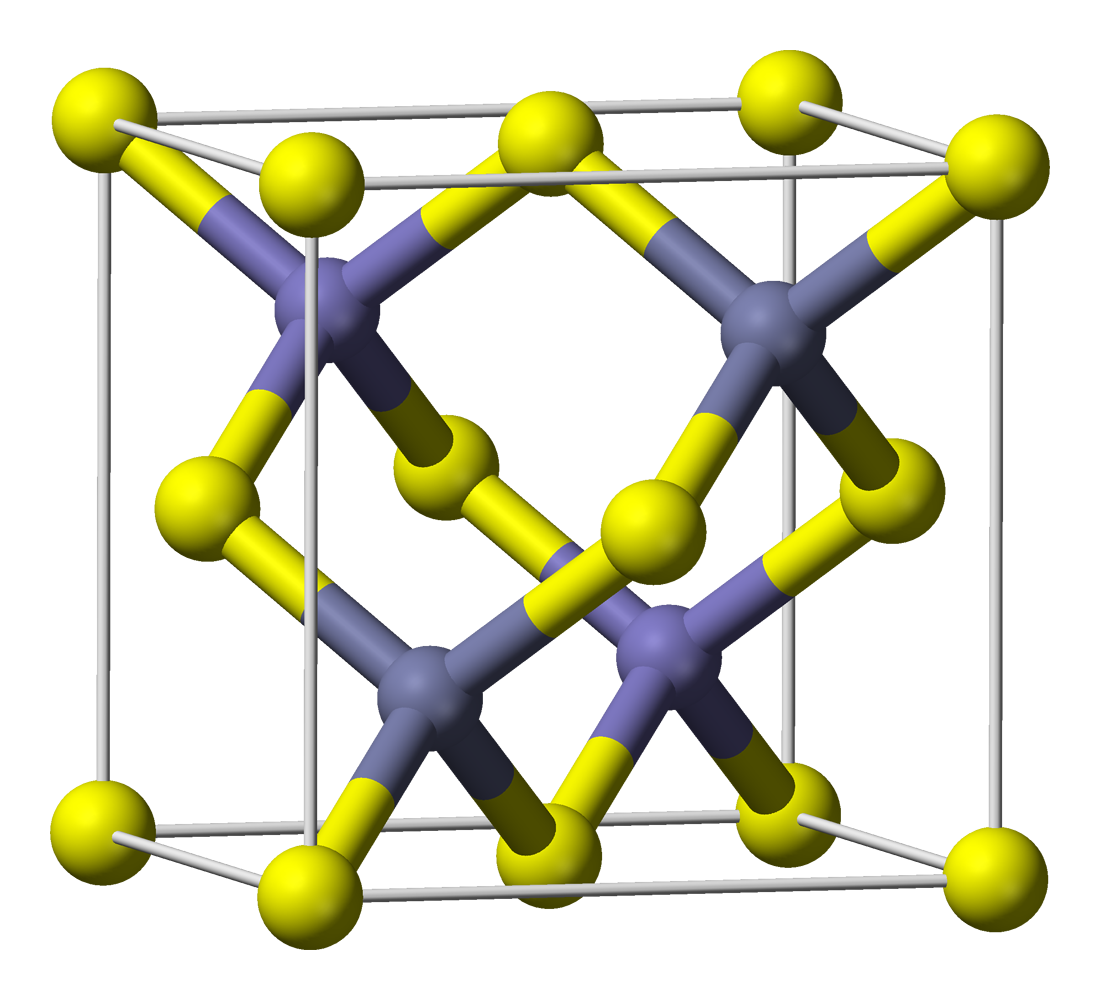
However, merely precipitating ZnS in the presence of Cu²⁺-ions does not yield phosphorescent ZnS:Cu. Zinc sulfide formed this way has a zinc blende structure (it is zinc blende) and it has a crystal lattice as shown here (image stolen from wikipedia):
The phosphorescent ZnS:Cu is first formed when the ZnS/CuS is heated to app 900°C under an inert atmosphere (or better yet, in an atmosphere of H₂S). In other words, it's not for kids.
Zinc sulfide is a semiconductor and when doped with Cu, electron holes are introduced. When exposed to (near-)UV light electrons are excited to the conduction band, and when they relax and recombine with the holes, light is given off.
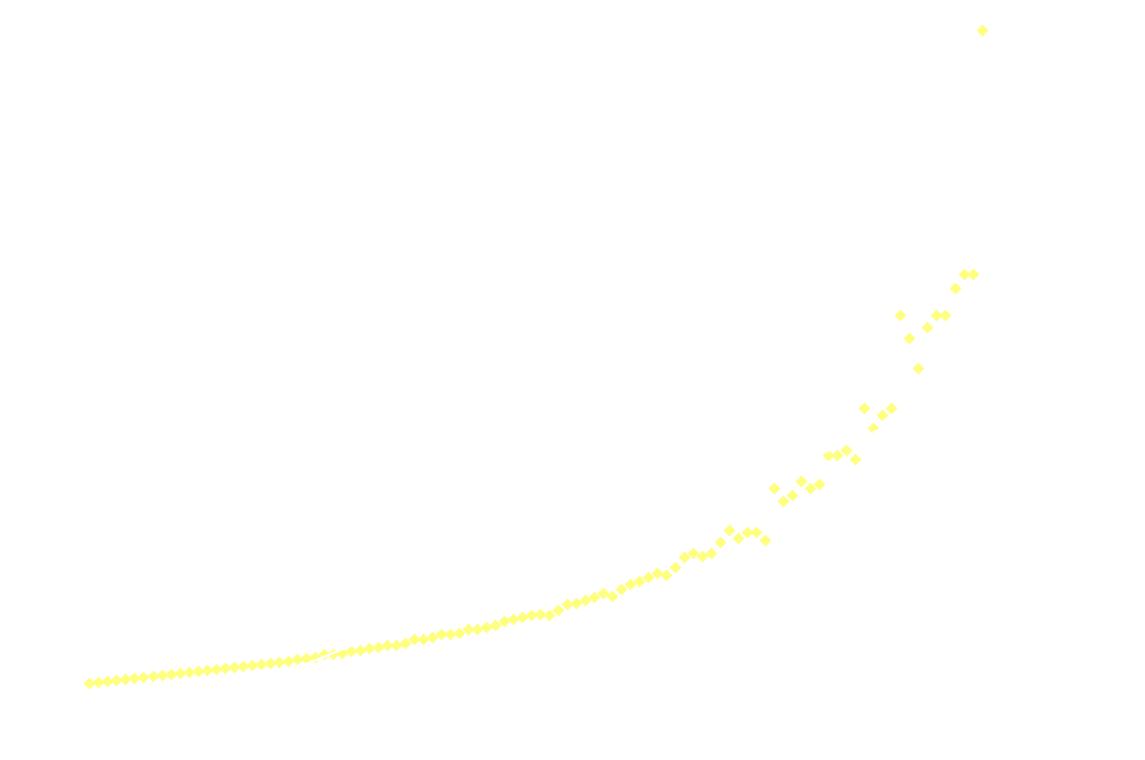
The kinetics of the decay can be studied by following the light intensity over time. In this experiment the Linear CCD module's SPI firmware was modified to power an LED (Osram Duris E5) which was placed behind a piece of a glow-in-the-dark star situated just in front of the CCD. The LED was turned off immediately (app 7.5 ms) before data collection.
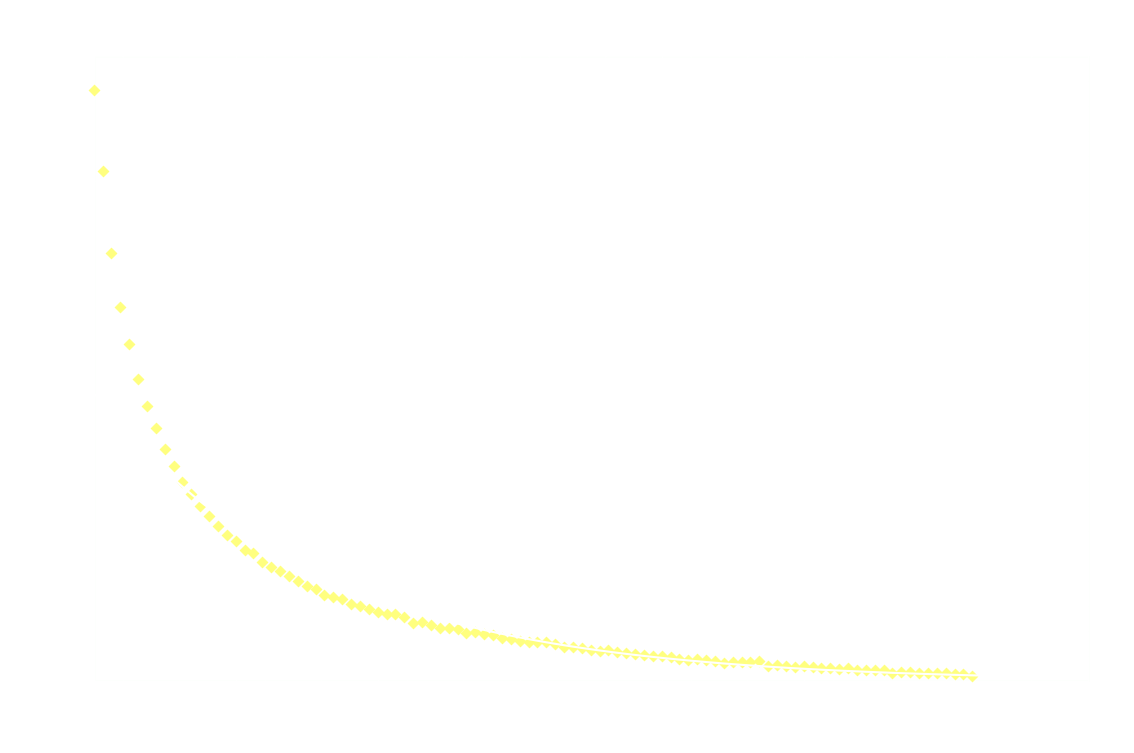
The following graph shows the light intensity as a function of time:

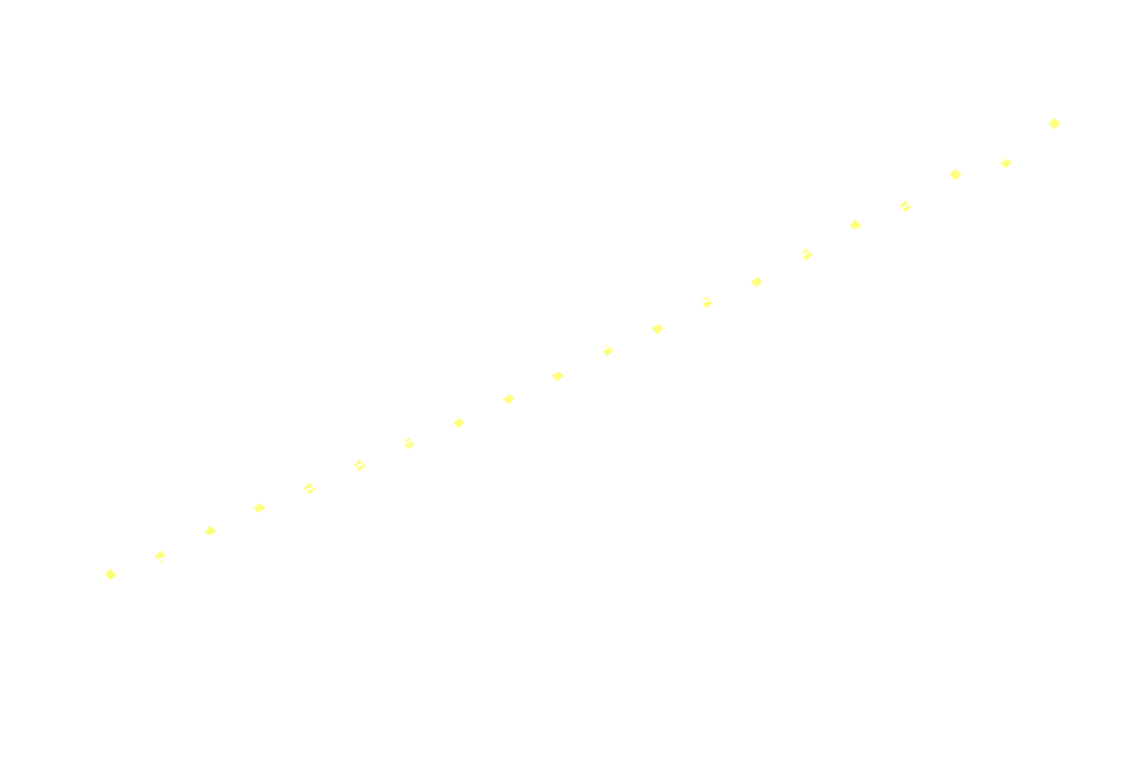
Surprisingly, the decay doesn't follow 1st order kinetics. The first 250ms it appears to follow 2nd order kinetics, as seen in the next graph:

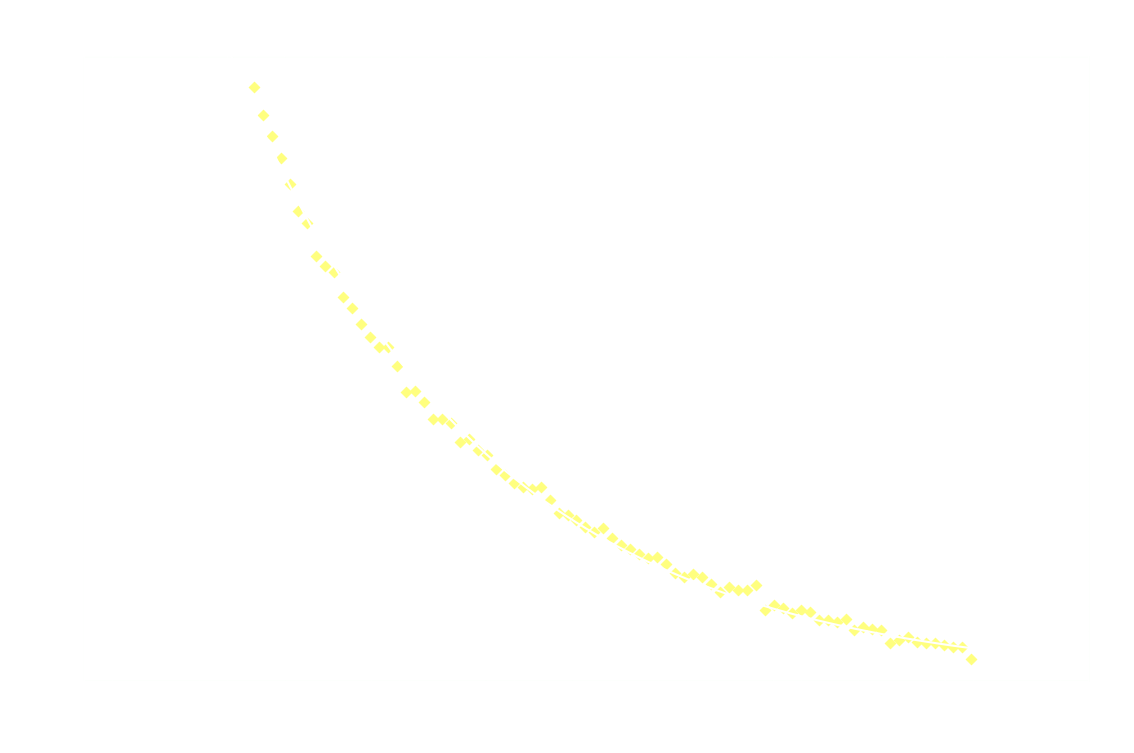
Hereon after, it's back to 1st order:

And just for good measure, here's the 2nd order model on the entire dataset:

Clearly 2nd order is not a good fit after the first 250ms.
It's not the LED-phosphor playing tricks, the LED-emission stops almost instantaneously after it's powered off (at least it's so fast, that it doesn't interfere with the measurements).
A similar investigation is presented in the following article, but the authors reach the opposite conclusion, that the decay is 1st order initially, and then becomes 2nd order:
Experiments with Glow-in-the-Dark Toys: Kinetics of Doped ZnS Phosphorescence, George C. Lisensky, Manish N. Patel, and Megan L. Reich, J. Chem. Educ., 1996, 73 (11), p 1048
-
Lab-procedure (I) Glow sticks
03/10/2018 at 15:34 • 0 commentsGlow sticks contain a diphenyl oxalate, hydrogen peroxide and a fluorophore, and a solvent of course.
The general reaction between diphenyl oxalate and hydrogen peroxide is:

The square shaped product 1,2-dioxetandione is not stable and immediately breaks down to CO₂:

This CO₂ is produced in an excited state and will immediately relax by emitting a photon in the UV-range:
The photon excites the fluorophore:
which remits a photon in the visible spectrum:
It's the fluorophore's emission spectrum that determines the colour of the glow stick.
In this procedure I'm using glow sticks for night fishing:
Each glow stick contains a glass ampoule with ca. 70mg of this particular diphenyl oxalate (R is pentyl):

and a fluorophor that could well be fluorescein.
The glass ampoule is surrounded by a viscous liquid that my IR-spectrometer identified as dibutyl phthalate. The phthalate contains enough H₂O₂ that my fingers turned snow-white after brief exposure to it.
Experimental:
A glow stick was carefully cut open with a scalpel and the glass ampoule was removed and placed in a beaker and rinsed with acetone. The clean ampoule was broken in a new beaker and the content dissolved in a few mL ethyl acetate. The solution was transferred to a 10 mL volumetric flask, which was filled to the mark with ethyl acetate.
Hydrogen peroxide (10 mL, 35%) was mixed with 30 mL ethyl acetate. After 2 min. of vigorous stirring the two-phase system was transferred to a separating funnel and the ethyl acetate was collected in a 50 mL conical flask. This yielded a solution of H₂O₂ in ethyl acetate with a concentration of app 2M (measured by titrating 200 µL of the solution in 10 mL 1M H₂SO₄ with 0,020M KMnO₄).
1.5 mL of the diphenyl oxalate solution was placed in the spectroscopic cell, after which 1.5 mL of the H₂O₂ solution was added and the measurements immediately started (integration time 2000 µs, measuring frequency 1 Hz, measuring time 50 s).

One will have to fiddle a bit with concentrations, integration time etc to get proper data, but worst of all also the base line.
Glowstick kinetics
Investigating the kinetics of chemiluminescent reactions using photometry.
 esben rossel
esben rossel